Abstract
Self-assembly of nanoparticles at solid–liquid interface could be promising to realize the assembled functions for various applications, such as rechargeable batteries, supercapacitors, and electrocatalysis. This review summarizes the self-assembly of the nanoparticles at solid–liquid interface according to the different driving forces of assembly, including hydrophilic–hydrophobic interactions, solvophobic and electrostatic interaction. To be specific, the self-assembly can be divided into the following two types: surfactant-assisted self-assembly and direct self-assembly of Janus particles (inorganic and amphiphilic copolymer-inorganic Janus nanoparticles). Using the emulsion stabilized by nanoparticles as the template, the self-assembly constructed by the interaction of the nanostructure unit (including metal, metal oxide, and semiconductor, etc.) not only possesses the characteristic of nanostructure unit, but also exhibits the excellent assembly performance in electrochemistry aspect. The application of these assemblies in the area of electrochemical capacitors is presented. Finally, the current research progress and perspectives toward the self-assembly of nanoparticles at stabilized solid–liquid interface are proposed.
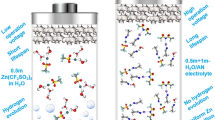
Graphical abstract

摘要
固液界面处纳米颗粒的自组装有望实现在可充电电池、超级电容器和电催化等领域的组装 功能。这篇综述总结了纳米粒子在固-液界面上的自组装可根据驱动力的不同, 分为亲水-疏 水相互作用、疏溶作用和静电相互作用。具体而言, 自组装可以分为以下两种类型: 表面 活性剂辅助自组装和Janus 粒子(无机和两亲性共聚物-无机Janus 纳米粒子)直接自组装。以纳米颗粒稳定的乳液为模板, 由纳米结构单元(包括金属、金属氧化物或半导体等)相互作 用构建的自组装不仅具有纳米结构单元的特性, 而且在宏观电化学方面也表现出优异的组 装特性, 文章也介绍了这些组件在电化学电容器领域的应用。最后, 提出了在固液界面处 纳米颗粒自组装的研究进展和展望。



Reproduced with permission from Ref. [35]. Copyright 2007, Wiley. c Self-assembled mechanism of multilayered plasmonic superstructures; d, e scanning electron microscope (SEM) images of self-assembled Au plasmonic superstructures. Reproduced with permission from Ref. [36]. Copyright 2015, Wiley. f Self-assembly process of hierarchical ZnMn2O4 hollow microspheres; g SEM image of self-supporting ZnMn2O4 microspheres. Reproduced with permission from Ref. [37]. Copyright 2014, Elsevier Publishing Group

Reproduced with permission from Ref. [38]. Copyright 2013, American Chemical Society

Copyright 2016, American Chemical Society. c Schematic illustration of formation mechanism of nanoscale colloidosomes: a key step is to form Janus bilayer on nanocrystal surfaces, where DTAB is dodecyltrimethylammonium bromide, ODE is octadecene; d 2D HAADF-STEM projection image from tilt series of half-filled superstructures. Reproduced with permission from Ref. [68]. Copyright 2016, American Chemical Society. e 3D self-assembly of Au@PS nanoparticles, where THF is tetrahydrofuran; f TEM image of Au@PS nanoparticles. Reproduced with permission from Ref. [69]. Copyright 2012, American Chemical Society

Reproduced with permission from Ref. [74]. Copyright 2013, American Chemical Society. Bright-field TEM images of hybrid micelles formed from: b PS51k-b-P4VP17k(PDP)2.0; c PS110k-b-P4VP107k(PDP)1.0 encapsulated of nanorods (content of 27 vol%, diameter of 7 nm, and length of 29 nm), where nanorods were grafted with mixed PS brushes (PS2k:PS12k = 1:1). Reproduced with permission from Ref. [75]. Copyright 2013, American Chemical Society. d Morphologies and nanorod concentrations. Reproduced with permission from Ref. [76]. Copyright 2010, Elsevier

Reproduced with permission from Ref. [87]. Copyright 2019, Wiley

Reproduced with permission from Ref. [100]. Copyright 2017, Wiley

Reproduced with permission from Ref. [107]. Copyright 2020, Elsevier

Reproduced with permission from Ref. [113]. Copyright 2018, Wiley
Similar content being viewed by others
References
Grzelczak M, Liz-Marza´n LM, Klajn R. Stimuli-responsive self-assembly of nanoparticles. Chem Soc Rev. 2019;48(5):1342. https://doi.org/10.1039/C8CS00787J.
Whitesides GM, Grzybowski B. Self-assembly at all scales. Science. 2002;295(5564):2418. https://doi.org/10.1126/science.1070821.
Shi Q, Dong DS, Si KJ, Sikdar D, Yap LW, Premaratne M, Cheng W. Shape transformation of constituent building blocks within self-assembled nanosheets and nano-origami. ACS Nano. 2018;12(2):1014. https://doi.org/10.1021/acsnano.7b08334.
Wang T, LaMontagne D, Lynch J, Zhuang J, Cao YC. Colloidal superparticles from nanoparticle assembly. Chem Soc Rev. 2013;42(7):2804. https://doi.org/10.1039/C2CS35318K.
Jain T, Roodbeen R, Reeler NE, Vosch T, Jensen KJ, Bjornholm T, Norgaard K. End-to-end assembly of gold nanorods via oligopeptide linking and surfactant control. J Colloid Interface Sci. 2012;376:83. https://doi.org/10.1016/j.jcis.2012.03.022.
Dong D, Yap LW, Smilgies DM, Si KJ, Shi Q, Cheng W. Two-dimensional gold trisoctahedron nanoparticle superlattice sheets: self-assembly, characterization and immunosensing applications. Nanoscale. 2018;10(11):5065. https://doi.org/10.1039/C7NR09443D.
Rao SY, Si KJ, Yap LW, Xiang Y, Cheng WL. Free-standing bi-layered nanoparticle superlattice nanosheets with asymmetric ionic transport behaviors. ACS Nano. 2015;9(11):11218. https://doi.org/10.1021/acsnano.5b04784.
Florea D, Wyss HM. Towards the self-assembly of anisotropic colloids: monodisperse oblate ellipsoids. J Colloid Interface Sci. 2014;416:30. https://doi.org/10.1016/j.jcis.2013.10.027.
Zhao Y, Shang L, Cheng Y, Gu Z. Spherical colloidal photonic crystals. Acc Chem Res. 2014;47(12):3632. https://doi.org/10.1021/ar500317s.
Lucio Isa KK, Mischa Müller JG, Marcus T, Erik R. Particle lithography from colloidal self-assembly at liquid-liquid interfaces. ACS Nano. 2010;4(10):5665. https://doi.org/10.1021/nn101260f.
Ng KC, Udagedara IB, Rukhlenko ID, Chen Y, Tang Y, Premaratne M, Cheng WL. Free-standing plasmonic-nanorod superlattice sheets. ACS Nano. 2012;6(1):925. https://doi.org/10.1021/nn204498j.
Si KJ, Chen Y, Shi Q, Cheng W. Nanoparticle superlattices: the roles of soft ligands. Adv Sci. 2018;5(1):1700179. https://doi.org/10.1002/advs.201700179.
Cui Y, Wei Q, Park H, Lieber CM. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science. 2001;293(5533):1289. https://doi.org/10.1126/science.1062711.
Duan XF, Huang Y, Cui Y, Wang JF, Lieber CM. Indium phosphide nanowires as building blocks for nanoscale electronic and optoelectronic devices. Nature. 2001;409:66. https://doi.org/10.1038/35051047.
Rymaruk MJ, Cunningham VJ, Brown SL, Williams CN, Armes SP. Oil-in-oil pickering emulsions stabilized by diblock copolymer nanoparticles. J Colloid Interface Sci. 2020;580:354. https://doi.org/10.1016/j.jcis.2020.07.010.
Liu Q, Sun Z, Santamarina JC. Self-assembled nanoparticle-coated interfaces: capillary pressure, shell formation and buckling. J Colloid Interface Sci. 2021;581:251. https://doi.org/10.1016/j.jcis.2020.07.110.
Lin Z, Zhang Z, Li Y, Deng Y. Magnetic nano-Fe3O4 stabilized pickering emulsion liquid membrane for selective extraction and separation. Chem Eng J. 2016;288:305. https://doi.org/10.1016/j.cej.2015.11.109.
Gao Q, Wang C, Liu H, Chen Y, Tong Z. Dual nanocomposite multi-hollow polymer microspheres prepared by suspension polymerization based on a multiple pickering emulsion. Polym Chem. 2010;1:75. https://doi.org/10.1039/B9PY00255C.
Zhou S, Bismarck A, Steinke JHG. Interconnected macroporous glycidyl methacrylate-grafted dextran hydrogels synthesized from hydroxyapatite nanoparticle stabilized high internal phase emulsion templates. J Mater Chem. 2012;22(36):18824. https://doi.org/10.1039/C2JM33294A.
Kempin MV, Stock S, Klitzing R, Kraume M, Drews A. Influence of particle type and concentration on the ultrafiltration behavior of nanoparticle stabilized Pickering emulsions and suspensions. Sep Purif Technol. 2020;252: 117457. https://doi.org/10.1016/j.seppur.2020.117457.
Wang ZG, Li N, Wang T, Ding BQ. Surface-guided chemical processes on self-assembled DNA nanostructures. Langmuir. 2018;34(49):14954. https://doi.org/10.1021/acs.langmuir.8b01060.
Niehues M, Engel S, Ravoo BJ. Photo-responsive self-assembly of plasmonic magnetic Janus nanoparticles. Langmuir. 2021;37(37):11123. https://doi.org/10.1021/acs.langmuir.1c01979.
Rival JV, Mymoona P, Lakshmi KM, Pradeep T, Hibu ES. Self-assembly of precision noble metal nanoclusters: hierarchical structural complexity, colloidal superstructures, and applications. Small. 2021;17(27):2005718. https://doi.org/10.1002/smll.202005718.
Bao Y, Zhang Y, Liu P, Ma J, Zhang W, Liu C, Simion D. Novel fabrication of stable pickering emulsion and latex by hollow silica nanoparticles. J Colloid Interface Sci. 2019;553:83. https://doi.org/10.1016/j.jcis.2019.06.008.
Venkataramani D, Tsulaia A, Amin S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv Colloid Interface Sci. 2020;283:102234. https://doi.org/10.1016/j.cis.2020.102234.
Aveyard R, Binks BP, Clint JH. Emulsions stabilized solely by colloidal particles. Adv Colloid Interface Sci. 2003;100:503. https://doi.org/10.1016/S0001-8686(02)00069-6.
Binks BP, Murakami R. Phase inversion of particle-stabilized materials from foams to dry water. Nat Mater. 2006;5(11):865. https://doi.org/10.1038/nmat1757.
Binks BP, Lumsdon SO. Influence of particle wettability on the type and stability of surfactant-free emulsions. Langmuir. 2000;16(23):8622. https://doi.org/10.1021/la000189s.
Khedr A, Striolo A. Self-assembly of mono and poly-dispersed nanoparticles on emulsion droplets: antagonistic vs. synergistic effects as a function of particle size. Phys Chem Chem Phys. 2020;22(39):22662. https://doi.org/10.1039/D0CP02588G.
Hils C, Schmelz J, Drechsler M, Schmalz H. Janus micelles by crystallization-driven self-assembly of an amphiphilic, double-crystalline triblock terpolymer. J Am Chem Soc. 2021;143(38):15582. https://doi.org/10.1021/jacs.1c08076.
Kim BS, Taton TA. Multicomponent nanoparticles via self-assembly with cross-linked block copolymer surfactants. Langmuir. 2007;23:2198. https://doi.org/10.1021/la062692w.
Eck W, Küller A, Grunze M, Völkel B, Gölzhäuser A. Freestanding nanosheets from crosslinked biphenyl self-assembled monolayers. Adv Mater. 2005;17(21):2583. https://doi.org/10.1002/adma.200500900.
Nikoobakht B, El Sayed MA. Evidence for bilayer assembly of cationic surfactants on the surface of gold nanorods. Langmuir. 2001;17:6368. https://doi.org/10.1021/la010530o.
Dinsmore AD, Hsu MF, Nikolaides MG, Marquez M, Bausch AR, Weitz DA. Colloidosomes: selectively permeable capsules composed of colloidal particles. Science. 2002;298(5595):1006. https://doi.org/10.1126/science.1074868.
Bai F, Wang D, Huo Z, Chen W, Liu L, Liang X, Chen C, Wang X, Peng Q, Li Y. A versatile bottom-up assembly approach to colloidal spheres from nanocrystals. Angew Chem Int Ed. 2007;119(35):6770. https://doi.org/10.1002/ange.200701355.
Liu D, Zhou F, Li C, Zhang T, Zhang H, Cai W, Li Y. Black gold: plasmonic colloidosomes with broadband absorption self-assembled from monodispersed gold nanospheres by using a reverse emulsion system. Angew Chem Int Ed. 2015;54(33):9596. https://doi.org/10.1002/anie.201503384.
Zhao M, Cai B, Ma Y, Cai H, Huang J, Pan X, He H, Ye Z. Self-assemble ZnMn2O4 hierarchical hollow microspheres into self-supporting architecture for enhanced biosensing performance. Biosens Bioelectron. 2014;61:443. https://doi.org/10.1016/j.bios.2014.05.051.
Lee SH, Yu SH, Lee JE, Jin A, Lee DJ, Lee N, Jo H, Shin K, Ahn TY, Kim YW, Choe H, Sung YE, Hyeon T. Self-assembled Fe3O4 nanoparticle clusters as high-performance anodes for lithium-ion batteries via geometric confinement. Nano Lett. 2013;13(9):4249. https://doi.org/10.1021/nl401952h.
Park J, An K, Hwang Y, Park JG, Noh HJ, Kim JY, Park JH, Hwang NM, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat Mater. 2004;3(12):891. https://doi.org/10.1038/nmat1251.
Buck MR, Biacchi AJ, Schaak RE. Insights into the thermal decomposition of Co(II) oleate for the shape-controlled synthesis of wurtzite-type CoO nanocrystals. Chem Mater. 2014;26(3):1492. https://doi.org/10.1021/cm4041055.
Joo J, Na HB, Yu T, Yu JH, Kim YW, Wu F, Zhang JZ, Hyeon T. Generalized and facile synthesis of semiconducting metal sulfide nanocrystals. J Am Chem Soc. 2003;125(36):11100. https://doi.org/10.1021/ja0357902.
Walther A, Muller AHE. Janus particles: synthesis, self-assembly, physical properties, and applications. Chem Rev. 2013;113(7):5194. https://doi.org/10.1021/cr300089t.
Chen D, Amstad E, Zhao CX, Cai L, Fan J, Chen Q, Hai M, Koehler S, Zhang H, Liang F, Yang Z, Weitz DA. Biocompatible amphiphilic hydrogel-solid dimer particles as colloidal surfactants. ACS Nano. 2017;11(12):11978. https://doi.org/10.1021/acsnano.7b03110.
Liu Y, Deng K, Yang J, Wu X, Fan X, Tang M, Quan Z. Shape-directed self-assembly of nano dumbbells into superstructure polymorphs. Chem Sci. 2020;11(16):4065. https://doi.org/10.1039/D0SC00592D.
Lu Y, Lin J, Wang L, Zhang L, Cai C. Self-assembly of copolymer micelles: higher-level assembly for constructing hierarchical structure. Chem Rev. 2020;120(9):4111. https://doi.org/10.1021/acs.chemrev.9b00774.
Tang Z, Gao L, Lin J, Cai C, Yao Y, Guerin G, Tian X, Lin S. Anchorage-dependent living supramolecular self-assembly of polymeric micelles. J Am Chem Soc. 2021;143(36):14684. https://doi.org/10.1021/jacs.1c06020.
Karayianni M, Pispas S. Block copolymer solution self-assembly: recent advances, emerging trends, and applications. J Polym Sci. 2021;59(17):1874. https://doi.org/10.1002/pol.20210430.
Zhu J, Hayward RC. Hierarchically structured microparticles formed by interfacial instabilities of emulsion droplets containing amphiphilic block copolymers. Angew Chem Int Ed. 2008;47(11):2113. https://doi.org/10.1002/ange.200704863.
Tagliabue A, Izzo L, Mella M. Out of equilibrium self-assembly of Janus nanoparticles: steering it from disordered amorphous to 2D patterned aggregates. Langmuir. 2016;32(48):12934. https://doi.org/10.1021/acs.langmuir.6b02715.
Gai Y, Lin Y, Song DP, Yavitt BM, Watkins JJ. Strong ligand-block copolymer interactions for incorporation of relatively large nanoparticles in ordered composites. Macromolecules. 2016;49(9):3352. https://doi.org/10.1021/acs.macromol.5b02609.
Ha JM, Lim SH, Dey J, Lee SJ, Lee MJ, Kang SH, Jin KS, Choi SM. Micelle-assisted formation of nanoparticle superlattices and thermally reversible symmetry transitions. Nano Lett. 2019;19(4):2313. https://doi.org/10.1021/acs.nanolett.8b04817.
Zubarev ER, Xu J, Sayyad A, Gibson JD. Amphiphilicity-driven organization of nanoparticles into discrete assemblies. J Am Chem Soc. 2006;128(47):15098. https://doi.org/10.1021/ja066708g.
Guo Y, Saei SH, Izumi CMS, Moffitt MG. Block copolymer mimetic self-assembly of inorganic nanoparticles. ACS Nano. 2011;5(4):3309. https://doi.org/10.1021/nn200450c.
Li X, Li H, Liu G, Deng Z, Wu S, Li P, Xu Z, Xu H, Chu PK. Magnetite-loaded fluorine-containing polymeric micelles for magnetic resonance imaging and drug delivery. Biomaterials. 2012;33(10):3013. https://doi.org/10.1016/j.biomaterials.2011.12.042.
Cao W, Xia S, Jiang X, Appold M, Opel M, Plank M, Schaffrinna R, Kreuzer LP, Yin S, Gallei M, Schwartzkopf M, Roth SV, Muller Buschbaum P. Self-assembly of large magnetic nanoparticles in ultrahigh molecular weight linear diblock copolymer films. ACS Appl Mater Interfaces. 2020;12(6):7557. https://doi.org/10.1021/acsami.9b20905.
Kao J, Xu T. Nanoparticle assemblies in supramolecular nanocomposite thin films: concentration dependence. J Am Chem Soc. 2015;137(19):6356. https://doi.org/10.1021/jacs.5b02494.
Yuan Q, Russell TP, Wang D. Self-assembly behavior of PS-b-P2VP block copolymers and carbon quantum dots at water/oil interfaces. Macromolecules. 2020;53(24):10981. https://doi.org/10.1021/acs.macromol.0c02422.
Wang M, Zhang M, Siegers C, Scholes GD, Winnik MA. Polymer vesicles as robust scaffolds for the directed assembly of highly crystalline nanocrystals. Langmuir. 2009;25(24):13703. https://doi.org/10.1021/la900523s.
Hickey RJ, Meng X, Zhang P, Park SJ. Low-dimensional nanoparticle clustering in polymer micelles and their transverse relaxivity rates. ACS Nano. 2013;7(7):5824. https://doi.org/10.1021/nn400824b.
Li W, Liu S, Deng R, Zhu J. Encapsulation of nanoparticles in block copolymer micellar aggregates by directed supramolecular assembly. Angew Chem Int Ed. 2011;50(26):5865. https://doi.org/10.1002/anie.201008224.
Choueiri RM, Klinkova A, Therien Aubin H, Rubinstein M, Kumacheva E. Structural transitions in nanoparticle assemblies governed by competing nanoscale forces. J Am Chem Soc. 2013;135(28):10262. https://doi.org/10.1021/ja404341r.
Sanwaria S, Horechyy A, Wolf D, Chu CY, Chen HL, Formanek P, Stamm M, Srivastava R, Nandan B. Helical packing of nanoparticles confined in cylindrical domains of a self-assembled block copolymer structure. Angew Chem Int Ed. 2014;53(34):9090. https://doi.org/10.1002/anie.201403565.
Singh S, Samanta P, Srivastava R, Horechyy A, Reuter U, Stamm M, Chen HL, Nandan B. Ligand displacement induced morphologies in block copolymer/quantum dot hybrids and formation of core-shell hybrid nanoobjects. Phys Chem Chem Phys. 2017;19(40):27651. https://doi.org/10.1039/C7CP04343K.
Jang SG, Khan A, Hawker CJ, Kramer EJ. Morphology evolution of PS-b-P2VP diblock copolymers via supramolecular assembly of hydroxylated gold nanoparticles. Macromolecules. 2012;45(3):1553. https://doi.org/10.1021/ma202391k.
Lin Y, Daga VK, Anderson ER, Gido SP, Watkins JJ. Nanoparticle-driven assembly of block copolymers: a simple route to ordered hybrid materials. J Am Chem Soc. 2011;133(17):6513. https://doi.org/10.1021/ja2003632.
Krack M, Hohenberg H, Kornowski A, Lindner P, Weller H, Förster S. Nanoparticle-loaded magnetophoretic vesicles. J Am Chem Soc. 2008;130(23):7315. https://doi.org/10.1021/ja077398k.
Deng R, Li H, Zhu J, Li B, Liang F, Jia F, Qu X, Yang Z. Janus nanoparticles of block copolymers by emulsion solvent evaporation induced assembly. Macromolecules. 2016;49(4):1362. https://doi.org/10.1021/acs.macromol.5b02507.
Yang Z, Altantzis T, Zanaga D, Bals S, Tendeloo GV, Pileni MP. Supracrystalline colloidal eggs: epitaxial growth and freestanding three-dimensional supracrystals in nanoscaled colloidosomes. J Am Chem Soc. 2016;138(10):3493. https://doi.org/10.1021/jacs.5b13235.
Sanchez Iglesias A, Grzelczak M, Altantzis T, Goris B, Perez Juste J, Bals S, Van Tendeloo G, Donaldson SH Jr, Chmelka BF, Israelachvili JN, Liz Marzan LM. Hydrophobic interactions modulate self-assembly of nanoparticles. ACS Nano. 2012;6(12):11059. https://doi.org/10.1021/nn3047605.
Zhu J, Hayward RC. Spontaneous generation of amphiphilic block copolymer micelles with multiple morphologies through interfacial instabilities. J Am Chem Soc. 2008;130(23):7496. https://doi.org/10.1021/ja801268e.
Ren M, Hou Z, Zheng X, Xu J, Zhu J. Electrostatic control of the three-dimensional confined assembly of charged block copolymers in emulsion droplets. Macromolecules. 2021;54(12):5728. https://doi.org/10.1021/acs.macromol.1c00575.
Yan N, Liu X, Zhu J, Zhu Y, Jiang W. Well-ordered inorganic nanoparticle arrays directed by block copolymer nanosheets. ACS Nano. 2019;13(6):6638. https://doi.org/10.1021/acsnano.9b00940.
Sánchez-Iglesias A, Claes N, Solis DM, Taboada JM, Bals S, Liz Marzan LM, Grzelczak M. Reversible clustering of gold nanoparticles under confinement. Angew Chem Int Ed. 2018;57(12):3183. https://doi.org/10.1002/ange.201800736.
Luo QJ, Hickey RJ, Park SJ. Controlling the location of nanoparticles in colloidal assemblies of amphiphilic polymers by tuning nanoparticle surface chemistry. ACS Macro Lett. 2013;2(2):107. https://doi.org/10.1021/mz3006044.
Li W, Zhang P, Dai M, He J, Babu T, Xu YL, Deng R, Liang R, Lu MH, Nie Z, Zhu J. Ordering of gold nanorods in confined spaces by directed assembly. Macromolecules. 2013;46(6):2241. https://doi.org/10.1021/ma400115z.
He L, Zhang L, Liang H. Mono- or bidisperse nanorods mixtures in diblock copolymers. Polymer. 2010;51(14):3303. https://doi.org/10.1016/j.polymer.2010.05.026.
El-Kady MF, Strong V, Dubin S, Kaner RB. Laser scribing of high-performance and flexible graphene-based electrochemical capacitors. Science. 2012;335(6074):1326. https://doi.org/10.1126/science.1216744.
Jiang SH, Ding J, Wang RH, Chen FY, Sun J, Deng YX, Li XL. Solvothermal-induced construction of ultra-tiny Fe2O3 nanoparticles/graphene hydrogels as binder-free high-capacitance anode for supercapacitors. Rare Met. 2021;40(12):3520. https://doi.org/10.1007/s12598-021-01739-8.
Simon P, Gogotsi Y. Materials for electrochemical capacitors. Nat Mater. 2008;7:845. https://doi.org/10.1142/9789814287005_0033.
Shen L, Yu L, Wu HB, Yu XY, Zhang X, Lou XW. Formation of nickel cobalt sulfide ball-in-ball hollow spheres with enhanced electrochemical pseudocapacitive properties. Nat Commun. 2015;6:6694. https://doi.org/10.1038/ncomms7694.
Yu L, Guan BY, Xiao W, Lou XW. Formation of yolk-shelled Ni-Co mixed oxide nanoprisms with enhanced electrochemical performance for hybrid supercapacitors and lithium-ion batteries. Adv Energy Mater. 2015;5(21):1500981. https://doi.org/10.1002/aenm.201500981.
Ren Y, Hardwick LJ, Bruce PG. Lithium intercalation into mesoporous anatase with an ordered 3D pore structure. Angew Chem Int Ed. 2010;122(14):2624. https://doi.org/10.1002/ange.200907099.
Ji HX, Zhao X, Qiao ZH, Jung J, Zhu YW, Lu YL, Zhang LL, MacDonald AH, Ruoff RS. Capacitance of carbon-based electrical double-layer capacitors. Nat Commun. 2014;5:3317. https://doi.org/10.1038/ncomms4317.
Wang FX, Wu XW, Yuan XH, Liu ZC, Zhang Y, Fu LJ, Zhu YS, Zhou QM, Wu YP, Huang W. Latest advances in supercapacitors: from new electrode materials to novel device designs. Chem Soc Rev. 2017;46:6816. https://doi.org/10.1039/C7CS00205J.
Chmiola J, Largeot C, Taberna PL, Simon P, Gogotsi Y. Monolithic carbide-derived carbon films for micro-supercapacitors. Science. 2010;328(5977):480. https://doi.org/10.1126/science.1184126.
Li H, Zhu Y, Dong S, Shen L, Chen Z, Zhang X, Yu G. Self-assembled Nb2O5 nanosheets for high energy-high power sodium ion capacitors. Chem Mater. 2016;28(16):5753. https://doi.org/10.1021/acs.chemmater.6b01988.
Huang J, Xiao YB, Peng ZY, Xu YZ, Li LB, Tan LC, Yuan K, Chen YW. Co3O4 supraparticle-based bubble nanofiber and bubble nanosheet with remarkable electrochemical performance. Adv Sci. 2019;6(12):1900107. https://doi.org/10.1002/advs.201900107.
Zhang JT, Hu H, Li Z, Lou XW. Double-shelled nanocages with cobalt hydroxide inner shell and layered double hydroxides outer shell as high-efficiency polysulfide mediator for lithium-sulfur batteries. Angew Chem Int Ed. 2016;128(12):4050. https://doi.org/10.1002/ange.201511632.
Xia BY, Yan Y, Li N, Wu HB, Lou XW, Wang X. A metal-organic framework-derived bifunctional oxygen electrocatalyst. Nat Energy. 2016;1:15006. https://doi.org/10.1038/nenergy.2015.6.
Hu H, Han L, Yu MZ, Wang ZY, Lou XW. Metal-organic-framework-engaged formation of Co nanoparticle-embedded carbon@Co9S8 double-shelled nanocages for efficient oxygen reduction. Energy Environ Sci. 2016;9:107. https://doi.org/10.1039/C5EE02903A.
Cai XJ, Gao W, Ma M, Wu MY, Zhang LL, Zheng YY, Chen HR, Shi JL. A Prussian blue-based core-shell hollow-structured mesoporous nanoparticle as a smart theranostic agent with ultrahigh pH-responsive longitudinal relaxivity. Adv Mater. 2015;27(41):6382. https://doi.org/10.1002/adma.201503381.
Hu L, Chen QW. Hollow/porous nanostructures derived from nanoscale metal-organic frameworks towards high performance anodes for lithium-ion batteries. Nanoscale. 2014;6(3):1236. https://doi.org/10.1039/C3NR05192G.
Zhang L, Wu HB, Lou XW. Metal-organic-frameworks-derived general formation of hollow structures with high complexity. J Am Chem Soc. 2013;135(29):10664. https://doi.org/10.1021/ja401727n.
Liu J, Wu C, Xiao DD, Kopold P, Gu L, van Aken PA, Maier J, Yu Y. MOF-derived hollow Co9S8 nanoparticles embedded in graphitic carbon nanocages with superior Li-ion storage. Small. 2016;12(17):2354. https://doi.org/10.1002/smll.201503821.
Wu RB, Wang DP, Rui XH, Liu B, Zhou K, Law AWK, Yan QY, Wei J, Chen Z. In-situ formation of hollow hybrids composed of cobalt sulfides embedded within porous carbon polyhedra/carbon nanotubes for high-performance lithium-ion batteries. Adv Mater. 2015;27(19):3038. https://doi.org/10.1002/adma.201500783.
Chen YM, Yu L, Lou XW. Hierarchical tubular structures composed of Co3O4 hollow nanoparticles and carbon nanotubes for lithium storage. Angew Chem Int Ed. 2016;55(20):5990–3. https://doi.org/10.1002/anie.201600133.
Han L, Yu XY, Lou XW. Formation of Prussian-blue-analog nanocages via a direct etching method and their conversion into Ni-Co-mixed oxide for enhanced oxygen evolution. Adv Mater. 2016;28(23):4601. https://doi.org/10.1002/adma.201506315.
Zou F, Hu XL, Li Z, Qie L, Hu CC, Zeng R, Jiang Y, Huang YH. MOF-derived porous ZnO/ZnFe2O4/C octahedra with hollow interiors for high-rate lithium-ion batteries. Adv Mater. 2014;26(38):6622–8. https://doi.org/10.1002/adma.201402322.
Yu L, Yang JF, Lou XW. Formation of CoS2 nanobubble hollow prisms for highly reversible lithium storage. Angew Chem Int Ed. 2016;128(43):13620. https://doi.org/10.1002/ange.201606776.
Guan BY, Lou XW. Complex cobalt sulfide nanobubble cages with enhanced electrochemical properties. Small Methods. 2017;1(7):1700158. https://doi.org/10.1002/smtd.201700158.
Zhao R, Wang M, Zhao D, Li H, Wang C, Yin L. Molecular-level heterostructures assembled from titanium carbide MXene and Ni-Co-Al layered double hydroxide nanosheets for All-solid-state flexible asymmetric high-energy supercapacitors. ACS Energy Lett. 2018;3(1):132. https://doi.org/10.1021/acsenergylett.7b01063.
Xie XQ, Zhao MQ, Anasori B, Maleski K, Ren CE, Li J, Byles BW, Pomerantseva E, Wang GX, Gogotsi Y. Porous heterostructured MXene/carbon nanotube composite paper with high volumetric capacity for sodium-based energy storage devices. Nano Energy. 2016;26:513. https://doi.org/10.1016/j.nanoen.2016.06.005.
Zhao RZ, Qian Z, Liu ZY, Zhao DY, Hui XB, Jiang GZ, Wang CX, Yin LW. Molecular-level heterostructures assembled from layered black phosphorene and Ti3C2 MXene as superior anodes for high-performance sodium ion batteries. Nano Energy. 2019;65:104037. https://doi.org/10.1016/j.nanoen.2019.104037.
Zhao RZ, Di HX, Hui XB, Zhao DY, Wang RT, Wang CX, Yin LW. Self-assembled Ti3C2 MXene and N-rich porous carbon hybrids as superior anodes for high-performance potassium-ion batteries. Energy Environ Sci. 2020;13:246. https://doi.org/10.1039/D1EE90043A.
Ling Z, Ren CE, Zhao MQ, Yang J, Giammarco JM, Qiu J, Barsoum MW, Gogotsi Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc Natl Acad Sci USA. 2014;111:16676. https://doi.org/10.1073/pnas.1414215111.
Hui XB, Ge XL, Zhao RZ, Li ZQ, Yin LW. Interface chemistry on MXene-based materials for enhanced energy storage and conversion performance. Adv Funct Mater. 2020;30(50):2005190. https://doi.org/10.1002/adfm.202005190.
Wu XM, Huang B, Wang QG, Wang Y. High energy density of two-dimensional MXene/NiCo-LDHs interstratification assembly electrode: understanding the role of interlayer ions and hydration. Chem Eng J. 2020;380:122456. https://doi.org/10.1016/j.cej.2019.122456.
Ding J, Hu WB, Paek ES, Mitlin D. Review of hybrid ion capacitors: from aqueous to lithium to sodium. Chem Rev. 2018;118:6457. https://doi.org/10.1021/acs.chemrev.8b00116.
Wang HW, Zhu CR, Chao DL, Yan QY, Fan HJ. Nonaqueous hybrid lithium-ion and sodium-ion capacitors. Adv Mater. 2017;29(46):1702093. https://doi.org/10.1002/adma.201702093.
Kang R, Zhu WQ, Li S, Zou BB, Wang LL, Li GC, Liu XH, Ng DHL, Qiu JX, Zhao Y, Qiao F, Lian JB. Fe2TiO5 nanochains as anode for high-performance lithium-ion capacitor. Rare Met. 2021;40(9):2424. https://doi.org/10.1007/s12598-020-01638-4.
Liu JL, Wang J, Xu CH, Jiang H, Li CZ, Zhang LL, Lin JY, Shen ZX. Advanced energy storage devices: basic principles, analytical methods, and rational materials design. Adv Sci. 2018;5(1):1700322. https://doi.org/10.1002/advs.201700322.
Wang YG, Song YF, Xia YY. Electrochemical capacitors: mechanism, materials, systems, characterization and applications. Chem Soc Rev. 2016;45:5925. https://doi.org/10.1039/C5CS00580A.
Hu ZL, Sayed S, Jiang T, Zhu XY, Lu C, Wang GL, Sun JY, Rashid A, Yan CL, Zhang L, Liu ZF. Self-assembled binary organic granules with multiple lithium uptake mechanisms toward high-energy flexible lithium-ion hybrid supercapacitors. Adv Energy Mater. 2018;8(30):1802273. https://doi.org/10.1002/aenm.201802273.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (Nos. 51772296, 5217020858, 51902016 and 21975015) and the Fundamental Research Funds for the Central Universities (Nos. buctrc201829 and buctrc201904).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Li, X., Chen, C., Niu, Q. et al. Self-assembly of nanoparticles at solid–liquid interface for electrochemical capacitors. Rare Met. 41, 3591–3611 (2022). https://doi.org/10.1007/s12598-022-02061-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02061-7