Abstract
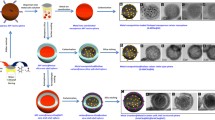
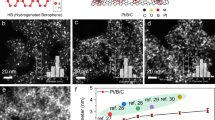
Small-sized bimetallic nanoparticles that possess numerous accessible metal sites and optimal geometric/electronic structures show great promise for advanced synergetic catalysis but remain synthetic challenge so far. Here, an universial synthetic method is developed for building a library of bimetallic nanoparticles on mesoporous sulfur-doped carbon supports, consisting of 24 combinations of 3 noble metals (that is, Pt, Rh, Ir) and 7 other metals, with average particle sizes ranging from 0.7 to 1.4 nm. The synthetic strategy is based on the strong metal-support interaction arising from the metal-sulfur bonding, which suppresses the metal aggregation during the H2-reduction at 700 °C and ensure the formation of small-sized and alloyed bimetallic nanoparticles. The enhanced catalytic properties of the ultrasmall bimetallic nanoparticles are demonstrated in the dehydrogenation of propane at high temperature and oxidative dehydrogenations of N-heterocycles.

Similar content being viewed by others
References
Alexeev, O. S.; Gates, B. C. Supported bimetallic cluster catalysts. Ind. Eng. Chem. Res.2003, 42, 1571–1587.
Buchwalter, P.; Rosé, J.; Braunstein, P. Multimetallic catalysis based on heterometallic complexes and clusters. Chem. Rev.2015, 115, 28–126.
Singh, A. K.; Xu, Q. Synergistic catalysis over bimetallic alloy nanoparticles. ChemCatChem2013, 5, 652–676.
Yang, X. F.; Wang, A. Q.; Qiao, B. T.; Li, J.; Liu, J. Y.; Zhang, T. Single-atom catalysts: A new frontier in heterogeneous catalysis. Acc. Chem. Res.2013, 46, 1740–1748.
Liu, L. C.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev.2018, 118, 4981–5079.
Mitchell, S.; Vorobyeva, E.; Pérez-Ramírez, J. Reactivity of single-atom heterogeneous catalysts: Unique and multifaceted. Angew. Chem., Int. Ed.2018, 57, 15316–15329.
Liu, P. X.; Zhao, Y.; Qin, R. X.; Mo, S. G.; Chen, G. X.; Gu, L.; Chevrier, D. M.; Zhang, P.; Guo, Q.; Zang, D. D. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science2016, 352, 797–800.
El-Sayed, M. A. Small is different: Shape-, size-, and composition-dependent properties of some colloidal semiconductor nanocrystals. Acc. Chem. Res.2004, 37, 326–333.
Yao, Y. G.; Huang, Z. N.; Xie, P. F.; Lacey, S. D.; Jacob, R. J.; Xie, H.; Chen, F. J.; Nie, A. M.; Pu, T. C.; Rehwoldt, M. et al. Carbothermal shock synthesis of high-entropy-alloy nanoparticles. Science2018, 359, 1489–1494.
Toshima, N. Polymer-protected bimetallic clusters. Preparation and application to catalysis. J. Macromol. Sci. A - Chem.1990, 27, 1225–1238.
Zhang, H. J.; Watanabe, T.; Okumura, M.; Haruta, M.; Toshima, N. Catalytically highly active top gold atom on palladium nanocluster. Nat. Mater.2012, 11, 49–52.
Kulkarni, A.; Gates, B. C. Spectroscopic elucidation of first steps of supported bimetallic cluster formation. Angew. Chem., Int. Ed.2009, 48, 9697–9700.
Chotisuwan, S.; Wittayakun, J.; Lobo-Lapidus, R. J.; Gates, B. C. MGO-supported cluster catalysts with Pt-Ru interactions prepared from Pt3Ru6(CO)21(μ3-H)(μ-h)3. Catal. Lett.2007, 115, 99–107.
Fung, A. S.; Kelley, M. J.; Koningsberger, D. C.; Gates, B. C. γ-Al2O3-supported Re-Pt cluster catalyst prepared from [Re2Pt(CO)12]: Characterization by extended X-ray absorption fine structure spectroscopy and catalysis of methylcyclohexane dehydrogenation. J. Am. Chem. Soc.1997, 119, 5877–5887.
Yang, J.; He, D. S.; Chen, W. X.; Zhu, W.; Zhang, H.; Ren, S.; Wang, X.; Yang, Q. H.; Wu, Y. E.; Li, Y. D. Bimetallic Ru-Co clusters derived from a confined alloying process within zeolite-imidazolate frameworks for efficient NH3 decomposition and synthesis. ACS Appl. Mater. Interfaces2017, 9, 39450–39455.
Iida, T.; Zanchet, D.; Ohara, K.; Wakihara, T.; Román-Leshkov, Y. Concerted bimetallic nanocluster synthesis and encapsulation via induced zeolite framework demetallation for shape and substrate selective heterogeneous catalysis. Angew. Chem., Int. Ed.2018, 57, 6454–6458.
Mao, J. J.; Li, J.; Pei, J. J.; Liu, Y.; Wang, D. S.; Li, Y. D. Structure regulation of noble-metal-based nanomaterials at an atomic level. Nano Today2019, 26, 164–175.
Wong, A.; Liu, Q.; Griffin, S.; Nicholls, A.; Regalbuto, J. R. Synthesis of ultrasmall, homogeneously alloyed, bimetallic nanoparticles on silica supports. Science2017, 358, 1427–1430.
Ding, K. L.; Cullen, D. A.; Zhang, L. B.; Cao, Z.; Roy, A. D.; Ivanov, I. N.; Cao, D. M. A general synthesis approach for supported bimetallic nanoparticles via surface inorganometallic chemistry. Science2018, 362, 560–564.
Wang, L.; Chen, M. X.; Yan, Q. Q.; Xu, S. L.; Chu, S. Q.; Chen, P.; Lin, Y.; Liang, H. W. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv.2019, 5, eaax6322.
Yan, Q. Q.; Wu, D. X.; Chu, S. Q.; Chen, Z. Q.; Lin, Y.; Chen, M. X.; Zhang, J.; Wu, X. J.; Liang, H. W. Reversing the charge transfer between platinum and sulfur-doped carbon support for electrocatalytic hydrogen evolution. Nat. Commun.2019, 10, 4977.
Choi, C. H.; Kim, M.; Kwon, H. C.; Cho, S. J.; Yun, S.; Kim, H. T.; Mayrhofer, K. J. J.; Kim, H.; Choi, M. Tuning selectivity of electrochemical reactions by atomically dispersed platinum catalyst. Nat. Commun.2016, 7, 10922.
Liu, B.; Yao, H. Q.; Song, W. Q.; Jin, L.; Mosa, I. M.; Rusling, J. F.; Suib, S. L.; He, J. Ligand-free noble metal nanocluster catalysts on carbon supports via “soft” nitriding. J. Am. Chem. Soc.2016, 138, 4718–4721.
Cheng, N. C.; Stambula, S.; Wang, D.; Banis, M. N.; Liu, J.; Riese, A.; Xiao, B. W.; Li, R. Y.; Sham, T. K.; Liu, L. M. et al. Platinum single-atom and cluster catalysis of the hydrogen evolution reaction. Nat. Commun.2016, 7, 13638.
Wu, Z. Y.; Xu, S. L.; Yan, Q. Q.; Chen, Z. Q.; Ding, Y. W.; Li, C.; Liang, H. W.; Yu, S. H. Transition metal-assisted carbonization of small organic molecules toward functional carbon materials. Sci. Adv.2018, 4, eaat0788.
Liang, H. W.; Brüller, S.; Dong, R. H.; Zhang, J.; Feng, X. L.; Müllen, K. Molecular metal-Nx centres in porous carbon for electrocatalytic hydrogen evolution. Nat. Commun.2015, 6, 7992.
Chen, L.; Cooper, A. C.; Pez, G. P.; Cheng, H. S. Mechanistic study on hydrogen spillover onto graphitic carbon materials. J. Phys. Chem. C2007, 111, 18995–19000.
Karim, W.; Spreafico, C.; Kleibert, A.; Gobrecht, J.; VandeVondele, J.; Ekinci, Y.; van Bokhoven, J. A. Catalyst support effects on hydrogen spillover. Nature2017, 541, 68–71.
Zhang, X.; Cui, G. Q.; Feng, H. S.; Chen, L. F.; Wang, H.; Wang, B.; Zhang, X.; Zheng, L. R.; Hong, S.; Wei, M. Platinum-copper single atom alloy catalysts with high performance towards glycerol hydrogenolysis. Nat. Commun.2019, 10, 5812.
Zhang, B.; Asakura, H.; Zhang, J.; Zhang, J. G.; De, S.; Yan, N. Stabilizing a platinum1 single-atom catalyst on supported phosphomolybdic acid without compromising hydrogenation activity. Angew. Chem., Int. Ed.2016, 55, 8319–8323.
Takahashi, M.; Koizumi, H.; Chun, W. J.; Kori, M.; Imaoka, T.; Yamamoto, K. Finely controlled multimetallic nanocluster catalysts for solvent-free aerobic oxidation of hydrocarbons. Sci. Adv.2017, 3, e1700101.
Wang, W. H.; Tian, X. L.; Chen, K.; Cao, G. Y. Synthesis and characterization of Pt-Cu bimetallic alloy nanoparticles by reverse micelles method. Colloids Surf. A2006, 273, 35–42.
Kim, N. R.; Shin, K.; Jung, I.; Shim, M.; Lee, H. M. Ag-Cu bimetallic nanoparticles with enhanced resistance to oxidation: A combined experimental and theoretical study. J. Phys. Chem. C2014, 118, 26324–26331.
Akporiaye, D.; Jensen, S.; Olsbye, U.; Rohr, F.; Rytter, E.; Rønnekleiv, M.; Spjelkavik, A. I. A novel, highly efficient catalyst for propane dehydrogenation. Ind. Eng. Chem. Res.2001, 40, 4741–4748.
Shen, J. Y.; Hill, J. M.; Watwe, R. M.; Spiewak, B. E.; Dumesic, J. A. Microcalorimetric, infrared spectroscopic, and DFT studies of ethylene adsorption on Pt/SiO2 and Pt-Sn/SiO2 catalysts. J. Phys. Chem. B1999, 103, 3923–3934.
Siri, G. J.; Ramallo-López, J. M.; Casella, M. L.; Fierro, J. L. G.; Requejo, F. G.; Ferretti, O. XPS and EXAFS study of supported PtSn catalysts obtained by surface organometallic chemistry on metals: Application to the isobutane dehydrogenation. Appl. Catal. A2005, 278, 239–249.
Cui, X.; Li, Y.; Bachmann, S.; Scalone, M.; Surkus, A. E.; Junge, K.; Topf, C.; Beller, M. Synthesis and characterization of iron-nitrogen-doped graphene/core-shell catalysts: Efficient oxidative dehydrogenation of N-heterocycles. J. Am. Chem. Soc.2015, 137, 10652–10658.
Chang, J. R.; Chang, S. L.; Lin, T. B. γ-Alumina-supported Pt catalysts for aromatics reduction: A structural investigation of sulfur poisoning catalyst deactivation. J. Catal.1997, 169, 338–346.
Chang, J. R.; Chang, S. L. Catalytic properties of γ-alumina- supported Pt catalysts for tetralin hydrogenation: Effects of sulfur-poisoning and hydrogen reactivation. J. Catal.1998, 176, 42–51.
Acknowledgements
This work was supported by the National Key Research and Development Program of China (Nos. 2018YFA0702001 and 2019YFA0307900), the National Natural Science Foundation of China (Nos. 21671184, 11874334, and 21872128), Youth Innovation Promotion Association CAS (No. 2020458), the Fundamental Research Funds for the Central Universities (Nos. WK2060190103 and WK2060030030), the Joint Funds from Hefei National Synchrotron Radiation Laboratory (No. KY2060000107), and the Recruitment Program of Thousand Youth Talents.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xu, SL., Shen, SC., Wei, ZY. et al. A library of carbon-supported ultrasmall bimetallic nanoparticles. Nano Res. 13, 2735–2740 (2020). https://doi.org/10.1007/s12274-020-2920-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-020-2920-8