Abstract
Purpose
The aim of this study is to explore the roles of β-catenin, decorin, septin-7, and S100A10 expression in colorectal cancer development.
Methods
Twenty-five BALB/c mice were divided into five groups; four groups were administrated N,N-dimethylhydrazine for 0, 10, 15, and 20 weeks, and one group was administrated normal saline for 20 weeks. The colons were collected for histopathological analysis. Protein samples prepared from the frozen colon tissues of mice treated with N,N-dimethylhydrazine for the different time points were evaluated using the isobaric tags for relative and absolute quantification (iTRAQ) labeling technique coupled with the 2D liquid chromatography–tandem mass spectrometry analysis. Based on the proteomic analysis results, immunohistochemical staining of β-catenin, decorin, septin-7, and S100A10 was performed in paraffin-embedded mice colorectal tissue, and 53 cases of human hereditary polyposis colorectal cancer samples.
Results
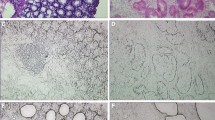
Colorectal cancer was observed in mice treated with N,N-dimethylhydrazine for 20 weeks, and adenomas were observed in mice subjected to the 10-, and 15-week treatments. Seventy-two differentially expressed proteins were involved in the development of cancer as per the iTRAQ and spectrometry analysis. In normal epithelium, adenoma, and cancer from human hereditary polyposis colorectal cancer, S100A10 expression (c2 = 100.989, P = 0.000) was highest in cancer, whereas decorin (c2 = 12.852, P = 0.002) and septin-7 (c2 = 66.519, P = 0.002) expressions were highest in the normal epithelium, which was confirmed via immunohistochemical staining.
Conclusions
The subcellular localization of β-catenin and decorin, septin-7, and S100A10 expressions are associated with the development of colorectal cancer in mice after N,N-dimethylhydrazine treatment and in human hereditary polyposis colorectal cancers.


Similar content being viewed by others
Abbreviations
- S100A10:
-
S100 Calcium-binding protein A10
- iTRAQ:
-
Isobaric tags for relative and absolute quantification
- FAP:
-
Familial adenomatous polyposis
- CRC:
-
Colorectal cancers
- APC:
-
Adenomatous polyposis coli
- DMH:
-
1,2-Dimethylhydrazine
- H&E:
-
Hematoxylin and eosin
- IHC:
-
Immunohistochemical
References
Kastrinos F, Mukherjee B, Tayob N, Wang F, Sparr J, Raymond VM, et al. Risk of pancreatic cancer in families with Lynch syndrome. JAMA. 2009;302(16):1790–5. https://doi.org/10.1001/jama.2009.1529.
Miladi-Abdennadher I, Amouri A, Ayadi L, Khabir A, Ellouze S, Tahri N, et al. A novel pathogenic germline mutation in the adenomatous polyposis coli gene in a Tunisian family with FAP. Fam Cancer. 2011;10(3):567–71. https://doi.org/10.1007/s10689-011-9451-0.
Song G, Yuan Y, Zheng F, Yang N, et al. Novel insertion mutation p.Asp610GlyfsX23 in APC gene causes familial adenomatous polyposis in Chinese families. Gene. 2013;516(2):204–8. https://doi.org/10.1016/j.gene.2012.12.077.
Soravia C, Berk T, Madlensky L, Mitri A, Cheng H, Gallinger S, et al. Genotype-phenotype correlations in attenuated adenomatous polyposis coli. Am J Hum Genet. 1998;62(6):1290–301. https://doi.org/10.1086/301883.
Zhang D, Fei F, Li S, Zhao Y, Yang Z, Qu J, et al. The role of beta-catenin in the initiation and metastasis of TA2 mice spontaneous breast cancer. J Cancer. 2017;8(11):2114–23. https://doi.org/10.7150/jca.19723.
Zhang Z, Liang S, Huang H, Wang D, Zhang X, Wu J, et al. A novel pathogenic large germline deletion in adenomatous polyposis coli gene in a Chinese family with familial adenomatous polyposis. Oncotarget. 2016;7(31):50392–400. https://doi.org/10.18632/oncotarget.10408.
Svitina H, Kyryk V, Skrypkina I, Kuchma M, Bukreieva T, Areshkov P, et al. Placenta-derived multipotent cells have no effect on the size and number of DMH-induced colon tumors in rats. Exp Ther Med. 2017;14(3):2135–47. https://doi.org/10.3892/etm.2017.4792.
Bekusova VV, Patsanovskii VM, Nozdrachev AD, Trashkov AP, Artemenko MR, Anisimov VN. Metformin prevents hormonal and metabolic disturbances and 1,2-dimethylhydrazine-induced colon carcinogenesis in non-diabetic rats. Cancer Biol Med. 2017;14(1):100–7. https://doi.org/10.20892/j.issn.2095-3941.2016.0088.
Kuznietsova HM, Luzhenetska VK, Kotlyar IP, Rybalchenko VK. Effects of 5-Amyno-4-(1,3-benzothyazol-2-yn)-1-(3-methoxyphenyl)-1,2-dihydro-3H-pyrrol-3-one intake on digestive system in a rat model of colon cancer. Sci World J. 2015;2015:376576. https://doi.org/10.1155/2015/376576.
Zhang S, Li M, Zhang D, Xu S, Wang X, Liu Z, et al. Hypoxia influences linearly patterned programmed cell necrosis and tumor blood supply patterns formation in melanoma. Lab Investig J Tech Methods Pathol. 2009;89(5):575–86. https://doi.org/10.1038/labinvest.2009.20.
Zhang S, Mercado-Uribe I, Xing Z, Sun B, Kuang J, Liu J. Generation of cancer stem-like cells through the formation of polyploid giant cancer cells. Oncogene. 2014;33(1):116–28. https://doi.org/10.1038/onc.2013.96.
Sun B, Zhang D, Zhang S, Zhang W, Guo H, Zhao X. Hypoxia influences vasculogenic mimicry channel formation and tumor invasion-related protein expression in melanoma. Cancer Lett. 2007;249(2):188–97. https://doi.org/10.1016/j.canlet.2006.08.016.
Zhang S, Mercado-Uribe I, Liu J. Tumor stroma and differentiated cancer cells can be originated directly from polyploid giant cancer cells induced by paclitaxel. Int J Cancer. 2014;134(3):508–18. https://doi.org/10.1002/ijc.28319.
Jia L, Zhang S, Ye Y, Li X, Mercado-Uribe I, Bast RC Jr, et al. Paclitaxel inhibits ovarian tumor growth by inducing epithelial cancer cells to benign fibroblast-like cells. Cancer Lett. 2012;326(2):176–82. https://doi.org/10.1016/j.canlet.2012.08.004.
Sun B, Zhang S, Zhang D, Li Y, Zhao X, Luo Y, et al. Identification of metastasis-related proteins and their clinical relevance to triple-negative human breast cancer. Clin Cancer Res. 2008;14(21):7050–9. https://doi.org/10.1158/1078-0432.CCR-08-0520.
Migliore L, Migheli F, Spisni R, Coppede F. Genetics, cytogenetics, and epigenetics of colorectal cancer. J Biomed Biotechnol. 2011;2011:792362. https://doi.org/10.1155/2011/792362.
Katono K, Sato Y, Jiang SX, Kobayashi M, Saito K, Nagashio R, et al. Clinicopathological significance of S100A10 expression in lung adenocarcinomas. Asian Pacific J Cancer Prev APJCP. 2016;17(1):289–94.
Hou Y, Yang L, Mou M, Hou Y, Zhang A, Pan N, et al. Annexin A2 regulates the levels of plasmin, S100A10 and Fascin in L5178Y cells. Cancer Investig. 2008;26(8):809–15. https://doi.org/10.1080/07357900801898664.
Phipps KD, Surette AP, O’Connell PA, Waisman DM. Plasminogen receptor S100A10 is essential for the migration of tumor-promoting macrophages into tumor sites. Can Res. 2011;71(21):6676–83. https://doi.org/10.1158/0008-5472.CAN-11-1748.
Tan Y, Ma SY, Wang FQ, Meng HP, Mei C, Liu A, et al. Proteomic-based analysis for identification of potential serum biomarkers in gallbladder cancer. Oncol Rep. 2011;26(4):853–9. https://doi.org/10.3892/or.2011.1353.
Ito Y, Arai K, Nozawa R, Yoshida H, Higashiyama T, Takamura Y, et al. S100A10 expression in thyroid neoplasms originating from the follicular epithelium: contribution to the aggressive characteristic of anaplastic carcinoma. Anticancer Res. 2007;27(4C):2679–83.
Mostowy S, Cossart P. Septins: the fourth component of the cytoskeleton. Nat Rev Mol Cell Biol. 2012;13(3):183–94. https://doi.org/10.1038/nrm3284.
Kinoshita A, Noda M, Kinoshita M. Differential localization of septins in the mouse brain. J Comp Neurol. 2000;428(2):223–39.
Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8(3):343–52. https://doi.org/10.1016/j.devcel.2004.12.005.
Jia ZF, Huang Q, Kang CS, Yang WD, Wang GX, Yu SZ, et al. Overexpression of septin 7 suppresses glioma cell growth. J Neurooncol. 2010;98(3):329–40. https://doi.org/10.1007/s11060-009-0092-1.
Igci YZ, Arslan A, Akarsu E, Erkilic S, Igci M, Oztuzcu S, et al. Differential expression of a set of genes in follicular and classic variants of papillary thyroid carcinoma. Endocr Pathol. 2011;22(2):86–96. https://doi.org/10.1007/s12022-011-9157-8.
Igci YZ, Erkilic S, Arslan A. Septin 7 immunoexpression in papillary thyroid carcinoma: a preliminary study. Pathol Res Pract. 2014;210(7):426–31. https://doi.org/10.1016/j.prp.2014.02.009.
Yoon AR, Hong J, Yun CO. Adenovirus-mediated decorin expression induces cancer cell death through activation of p53 and mitochondrial apoptosis. Oncotarget. 2017;8(44):76666–85. https://doi.org/10.18632/oncotarget.20800.
Bostrom P, Sainio A, Eigeliene N, Jokilammi A, Elenius K, Koskivuo I, et al. Human metaplastic breast carcinoma and decorin. Cancer Microenviron. 2017. https://doi.org/10.1007/s12307-017-0195-8.
Neill T, Schaefer L, Iozzo RV. Decorin as a multivalent therapeutic agent against cancer. Adv Drug Deliv Rev. 2016;97:174–85. https://doi.org/10.1016/j.addr.2015.10.016.
Funding
This work was supported in part by Grants from the National Science Foundation of China (#81472729 and #81672426), the foundation of Tianjin Health Bureau (15KG112) and the foundation of committee on science and technology of Tianjin (17ZXMFSY00120), and foundation of Tianjin Union Medical Center (2016YJ025).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
This study has been approved by Tianjin Union Medical Center’s ethics committee and has been performed according to the ethical standards laid down in the 1964 Declaration of Helsinki.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, G., Fei, F., Qu, J. et al. iTRAQ-based proteomic analysis of DMH-induced colorectal cancer in mice reveals the expressions of β-catenin, decorin, septin-7, and S100A10 expression in 53 cases of human hereditary polyposis colorectal cancer. Clin Transl Oncol 21, 220–231 (2019). https://doi.org/10.1007/s12094-018-1912-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-018-1912-6