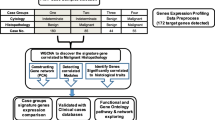
Abstract
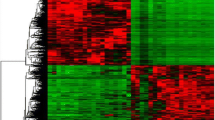
Fine-needle aspiration biopsy (FNA) is currently the best initial diagnostic test for evaluation of a thyroid nodule. FNA cytology cannot discriminate between benign and malignant thyroid nodules in up to 30% of thyroid nodules. Therefore, an adjunct to FNA is needed to clarify these lesions as benign or malignant. Using differential display-polymerase chain reaction method, the gene expression differences between follicular and classic variants of papillary thyroid carcinoma (PTC) and benign thyroid nodules were evaluated in a group of 42 patients. Computational gene function analyses via Cytoscape, FuncBASE, and GeneMANIA led us to a functional network of 17 genes in which a core sub-network of five genes coexists. Although the exact mechanisms underlying in thyroid cancer biogenesis are not currently known, our data suggest that the pattern of transformation from healthy cells to cancer cells of PTC is different in follicular variant than in classic variant.





Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics. CA Cancer J Clin. 58:71–96, 2008.
DeLellis RA. Pathology and genetics of thyroid carcinoma. Journal of Surgical Oncology 94:662–9, 2006.
Fagin JA, Mitsiades N. Molecular pathology of thyroid cancer: diagnostic and clinical implications. Best Practice & Research Clinical Endocrinology & Metabolism 22:955–69, 2008.
Barden CB, Shister KW, Zhu B, Guiter G, Greenblatt DY, Zeiger MA, Fahey TJ 3rd. Classification of follicular thyroid tumors by molecular signature: results of gene profiling. Clinical Cancer Research 9:1792–800, 2003.
Gharib H, Goellner JR. Fine-needle aspiration biopsy of the thyroid: an appraisal. Ann Intern Med.118:282–9, 1993
Shibru D, Chung KW, Kebebew E. Recent developments in the clinical application of thyroid cancer biomarkers. Current Opinion in Oncology 20:13–8, 2008.
Kato MA, Fahey TJ 3rd. Molecular markers in thyroid cancer diagnostics. Surgical Clinics of North America 89:1139–55, 2009.
Eszlinger M, Paschke R. Molecular fine-needle aspiration biopsy diagnosis of thyroid nodules by tumor specific mutations and gene expression patterns. Molecular and Celular Endocrinology 322:29–37, 2010.
Lee KY, Huang SM, Li S, Kim JM. Identification of differentially expressed genes in papillary thyroid cancers. Yonsei Medical Journal 50:60–7, 2009.
Liang P, Pardee AB. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257:967–71, 1992.
Sturtevant J. Applications of differential-display reverse transcription-PCR to molecular pathogenesis and medical mycology. Clinical Microbiology Reviews 13:408–27, 2000.
Chang KC, Komm B, Arnold NB, Korc M. The application of differential display as a gene profiling tool. Methods in Molecular Bioloy 383:31–40, 2007.
http://lukemiller.org/journal/2007/08/quantifying-western-blots-without.html. Quantifying western blots without expensive commercial quantification software. (Access date: 05 October 2010)
Nikiforova MN, Nikiforov YE. Molecular diagnostics and predictors in thyroid cancer. Thyroid 19:1351–61, 2009.
Liang P. Factors ensuring successful use of differential display. Methods 16:361–4, 1998.
Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Research 25:3389–402, 1997.
Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, ed. Bioinformatics Methods and Protocols: Methods in Molecular Biology. New Jersey: Humana Press, 2000; 365–386.
Cline MS, Smoot M, Cerami E, Kuchinsky A, Landys N, Workman C, Christmas R, Avila-Campilo I, Creech M, Gross B, Hanspers K, Isserlin R, Kelley R, Killcoyne S, Lotia S, Maere S, Morris J, Ono K, Pavlovic V, Pico AR, Vailaya A, Wang PL, Adler A, Conklin BR, Hood L, Kuiper M, Sander C, Schmulevich I, Schwikowski B, Warner GJ, Ideker T, Bader GD. Integration of biological networks and gene expression data using Cytoscape. Nature Protocols 2:2366–82, 2007.
Beaver JE, Tasan M, Gibbons FD, Tian W, Hughes TR, Roth FP. FuncBase: a resource for quantitative gene function annotation. Bioinformatics 26:1806–7, 2010.
Mostafavi S, Ray D, Warde-Farley D, Grouios C, Morris Q. GeneMANIA: a real-time multiple association network integration algorithm for predicting gene function. Genome Biology Supplements 1 9:S4, 2008.
Tye BK. MCM proteins in DNA replication. Annual Review of Biochemistry 68:649–86, 1999.
Zhu M, Wang F, Yan F, Yao PY, Du J, Gao X, Wang X, Wu Q, Ward T, Li J, Kioko S, Hu R, Xie W, Ding X, Yao X. Septin 7 interacts with centromere-associated protein E and is required for its kinetochore localization. The Journal of Biological Chemistry 283:18916–25, 2008.
Lee YS, Ha SA, Kim HJ, Shin SM, Kim HK, Kim S, Kang CS, Lee KY, Hong OK, Lee SH, Kwon HS, Cha BY, Kim JW. Minichromosome maintenance protein 3 is a candidate proliferation marker in papillary thyroid carcinoma. Experimental and Molecular Pathology 88:138–42, 2010.
Giaginis C, Vgenopoulou S, Vielh P, Theocharis S. MCM proteins as diagnostic and prognostic tumor markers in the clinical setting. Histology and Histopathology 25:351–70, 2010.
Kebebew E, Peng M, Reiff E, Duh QY, Clark OH McMillan A. Diagnostic and prognostic value of cell-cycle regulatory genes in malignant thyroid neoplasms. World Journal of Surgery 30:767–74, 2006.
Madine MA, Swietlik M, Pelizon C, Romanowski P, Mills AD, Laskey RA The roles of the MCM, ORC, and Cdc6 proteins in determining the replication competence of chromatin in quiescent cells. Journal of Structural Biology 129:198–210, 2000.
Peter HJ, Gerber H, Studer H, Smeds S. Pathogenesis of heterogeneity in human multinodular goiter. A study on growth and function of thyroid tissue transplanted onto nude mice. The Journal of Clinical Investigation 76:1992–2002, 1985.
Salabè GB. Pathogenesis of thyroid nodules: histological classification?. Biomedicine & Pharmacotherapy 55:39–53, 2001.
Thomas GA, Williams D, Williams ED. Clonal origin of thyroid tumours. In: Wynford-Thomas D, Williams ED, ed. Thyroid tumours. Edinburg: Churchill Livingstone, 1989; 38–56.
Zhang P, Zuo H, Ozaki T, Nakagomi N, Kakudo K. Cancer stem cell hypothesis in thyroid cancer. Pathology International 56:485–9, 2006.
Sertel S, Eichhorn T, Sieber S, Sauer A, Weiss J, Plinkert PK, Efferth T. Factors determining sensitivity or resistance of tumor cell lines towards artesunate. Chemico-Biological Interactions 185:42–52, 2010.
Chevillard S, Ugolin N, Vielh P, Ory K, Levalois C, Elliott D, Clayman GL, El-Naggar AK. Gene expression profiling of differentiated thyroid neoplasms: diagnostic and clinical implications. Clinical Cancer Research 10:6586–97, 2004.
Yulug IG, See CG, Fisher EM, Ylug IG. The DAD1 protein, whose defect causes apoptotic cell death, maps to human chromosome 14. Genomics 26:433–5, 1995.
Kulke MH, Freed E, Chiang DY, Philips J, Zahrieh D, Glickman JN, Shivdasani RA. High-resolution analysis of genetic alterations in small bowel carcinoid tumors reveals areas of recurrent amplification and loss. Genes Chromosomes Cancer 47:591–603, 2008.
He H, Nagy R, Liyanarachchi S, Jiao H, Li W, Suster S, Kere J, de la Chapelle A. A susceptibility locus for papillary thyroid carcinoma on chromosome 8q24. Cancer Research 69:625–31, 2009.
Kouniavsky G, Zeiger MA. Thyroid tumorigenesis and molecular markers in thyroid cancer. Current Opinion in Oncology 22:23–9, 2010.
Yotov WV, St-Arnaud R. Mapping of the human gene for the alpha-NAC/1.9.2 (NACA/1.9.2) transcriptional coactivator to Chromosome 12q23-24.1. Mammalian Genome 7:163–4, 1996.
Loeffen JL, Triepels RH, van den Heuvel LP, Schuelke M, Buskens CA, Smeets RJ, Trijbels JM, Smeitink JA. cDNA of eight nuclear encoded subunits of NADH:ubiquinone oxidoreductase: human complex I cDNA characterization completed. Biochemical and Biophysical Research Communications 253:415–22, 1998.
Woerner SM, Kloor M, Mueller A, Rueschoff J, Friedrichs N, Buettner R, Buzello M, Kienle P, Knaebel HP, Kunstmann E, Pagenstecher C, Schackert HK, Möslein G, Vogelsang H, von Knebel Doeberitz M, Gebert JF, German HNPCC Consortium. Microsatellite instability of selective target genes in HNPCC-associated colon adenomas. Oncogene 24:2525–35, 2005.
Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Modern Pathology 21(2):S37–43, 2008.
Jackson RS 2nd, Cho YJ, Liang P. TIS11D is a candidate pro-apoptotic p53 target gene. Cell Cycle 5:2889–93, 2006.
Finley DJ, Arora N, Zhu B, Gallagher L, Fahey TJ 3rd. Molecular profiling distinguishes papillary carcinoma from benign thyroid nodules. Journal of Clinical Endocrinology & Metabolism 89:3214–23, 2004.
Finley DJ, Zhu B, Barden CB, Fahey TJ 3rd. Discrimination of benign and malignant thyroid nodules by molecular profiling. Annals of Surgery 240:425–36; discussion 436–7, 2004.
Chuikov S, Kurash JK, Wilson JR, Xiao B, Justin N, Ivanov GS, McKinney K, Tempst P, Prives C, Gamblin SJ, Barlev NA, Reinberg D. Regulation of p53 activity through lysine methylation. Nature 432:353–60, 2004.
Huang J, Perez-Burgos L, Placek BJ, Sengupta R, Richter M, Dorsey JA, Kubicek S, Opravil S, Jenuwein T, Berger SL. Repression of p53 activity by Smyd2-mediated methylation. Nature 444:629–32, 2006.
Komatsu S, Imoto I, Tsuda H, Kozaki KI, Muramatsu T, Shimada Y, Aiko S, Yoshizumi Y, Ichikawa D, Otsuji E, Inazawa J. Overexpression of SMYD2 relates to tumor cell proliferation and malignant outcome of esophageal squamous cell carcinoma. Carcinogenesis 30:1139–46, 2009.
Yin Y, Liu YX, Jin YJ, Hall EJ, Barrett JC. PAC1 phosphatase is a transcription target of p53 in signalling apoptosis and growth suppression. Nature 422:527–31, 2003.
Acknowledgments
This study was supported by a research project (TF-09.12, 2009) from University of Gaziantep, Scientific Research Projects (BAP).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Igci, Y.Z., Arslan, A., Akarsu, E. et al. Differential Expression of a Set of Genes in Follicular and Classic Variants of Papillary Thyroid Carcinoma. Endocr Pathol 22, 86–96 (2011). https://doi.org/10.1007/s12022-011-9157-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12022-011-9157-8