Abstract
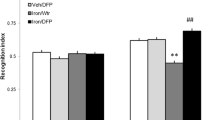
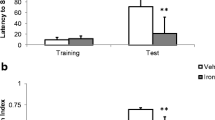
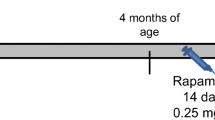
Iron accumulation has been associated with the pathogenesis of neurodegenerative diseases and memory decline. As previously described by our research group, iron overload in the neonatal period induces persistent memory deficits and increases oxidative stress and apoptotic markers. The neuronal insult caused by iron excess generates an energetic imbalance that can alter glutamate concentrations and thus trigger excitotoxicity. Drugs that block glutamatergic receptor eligibly mitigate neurotoxicity; among them is perampanel (PER), a reversible AMPA receptor (AMPAR) antagonist. In the present study, we sought to investigate the neuroprotective effects of PER in rats subjected to iron overload in the neonatal period. Recognition and aversive memory were evaluated, AMPAR subunit phosphorylation, as well as the relative expression of genes such as GRIA1, GRIA2, DLG4, and CAC, which code proteins involved in AMPAR anchoring. Male rats received vehicle or carbonyl iron (30 mg/kg) from the 12th to the 14th postnatal day and were treated with vehicle or PER (2 mg/kg) for 21 days in adulthood. The excess of iron caused recognition memory deficits and impaired emotional memory, and PER was able to improve the rodents’ memory. Iron increased the phosphorylation of GLUA1 subunit, which was reversed by PER. Furthermore, iron overload increased the expression of the GRIA1 gene and decreased the expression of the DLG4 gene, demonstrating the influence of metal accumulation on the metabolism of AMPAR. These results suggest that iron can interfere with AMPAR functionality, through altered phosphorylation of its subunits, and the expression of genes that code for proteins critically involved in the assembly and anchoring of AMPAR. The blockade of AMPAR with PER is capable of partially reversing the cognitive deficits caused by iron overload.





Similar content being viewed by others
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Murphy RJ, Buenzli PR, Baker RE, Simpson MJ (2020) Mechanical cell competition in heterogeneous epithelial tissues. Bull Math Biol 82(10):130. https://doi.org/10.1007/s11538-020-00807-x
Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G (2013) The hallmarks of aging. Cell 153:1194–1217. https://doi.org/10.1016/j.cell.2013.05.039
Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES et al (2014) Geroscience: linking aging to chronic disease. Cell 159:709–713. https://doi.org/10.1016/j.cell.2014.10.039
Ferreira B, Mendes F, Osorio N, Caseiro A, Gabriel A, Valado A (2013) Glutathione in multiple sclerosis. Br J Biomed Sci 70:75–79. https://doi.org/10.1080/09674845.2013.11669939
Zhu W, Xie W, Pan T, Xu P, Fridkin M, Zheng H et al (2007) Prevention and restoration of lactacystin-induced nigroestriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J 21:3835–3844. https://doi.org/10.1096/fj.07-8386com
Huat TJ, Camats-Perna J, Newcombe EA, Valmas N, Kitazawa M, Medeiros R (2019) Metal toxicity links to Alzheimer’s disease and neuroinflammation. J Mol Biol 431(9):1843–1868. https://doi.org/10.1016/j.jmb.2019.01.018
Lan AP, Chen J, Chai ZF, Hu Y (2016) The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 29(4):665–678. https://doi.org/10.1007/s10534-016-9942-4
Peng Y, Chang X, Lang M (2021) Iron homeostasis disorder and Alzheimer’s disease. Int J Mol Sci 22(22):12442. https://doi.org/10.3390/ijms222212442
Schröder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T (2001) Memory deficits in adult rats following postnatal iron administration. Behav Brain Res 124(1):77–85. https://doi.org/10.1016/s0166-4328(01)00236-4
Figueiredo LS, de Freitas BS, Garcia VA, Dargél VA, Köbe LM, Kist LW et al (2016) Iron loading selectively increases hippocampal levels of ubiquitinated proteins and impairs hippocampus-dependent memory. Mol Neurobiol 53:6228–6239. https://doi.org/10.1007/s12035-015-9514-6
de Lima MN, Polydoro M, Laranja DC, Bonatto F, Bromberg E, Moreira JC et al (2005) Recognition memory impairment and brain oxidative stress induced by postnatal iron administration. Eur J Neurosci 21:2521–2528. https://doi.org/10.1111/j.1460-9568.2005.04083.x
Miwa CP, de Lima MN, Scalco F, Vedana G, Mattos R, Fernandez LL et al (2011) Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox Res 19:527–535. https://doi.org/10.1007/s12640-010-9181-3
da Silva VK, de Freitas BS, da Silva DA, Nery LR, Falavigna L, Ferreira RD et al (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49:222–233. https://doi.org/10.1007/s12035-013-8514-7
Uberti VH, de Freitas BS, Molz P, Bromberg E, Schröder N (2019) Iron overload impairs autophagy: effects of rapamycin in ameliorating iron-related memory deficits. Mol Neurobiol 57(2):1044–1054. https://doi.org/10.1007/s12035-019-01794-4
Mythri RB, Venkateshappa C, Harish G, Mahadevan A, Muthane UB, Yasha TC (2011) Evaluation of markers of oxidative stress, antioxidant function and astrocytic proliferation in the striatum and frontal cortex of Parkinson’s disease brains. Neurochem Res 36:1452–1463. https://doi.org/10.1007/s11064-011-0471-9
Castellani RJ, Siedlak SL, Perry G, Smith MA (2000) Sequestration of iron by Lewy bodies in Parkinson’s disease. Acta Neuropathol 100:111–114. https://doi.org/10.1007/s004010050001
Cao YY, Wu LL, Li XN, Yuan YL, Zhao WW, Qi JX, Zhao XY, Ward N et al (2023) Molecular mechanisms of AMPA receptor trafficking in the nervous system. J Int J Mol Sci 25(1):111. https://doi.org/10.3390/ijms25010111
Penn AC, Zhang CL, Georges F, Royer L, Breillat C, Hosy E, Petersen JD, Humeau Y et al (2017) Hippocampal LTP and contextual learning require surface diffusion of AMPA receptors. Nature 549:384–388. https://doi.org/10.1038/nature23658
Bonini JS, Rodrigues L, Kerr DS, Bevilaqua LR, Cammarota M, Izquierdo I (2003) AMPA/kainate and group-I metabotropic receptor antagonists infused into different brain areas impair memory formation of inhibitory avoidance in rats. Behav Pharmacol 14(2):161–166. https://doi.org/10.1097/00008877-200303000-00008
Marcondes LA, Nachtigall EG, Zanluchi A, de Carvalho MJ, Izquierdo I, Furini CRG (2020) Involvement of medial prefrontal cortex NMDA and AMPA/kainate glutamate receptors in social recognition memory consolidation. Neurobiol Learn Mem 168:107153. https://doi.org/10.1016/j.nlm.2019.107153
Whitehead G, Regan P, Whitcomb DJ, Cho K (2017) Ca2+-permeable AMPA receptor: a new perspective on amyloid-beta mediated pathophysiology of Alzheimer’s disease. Neuropharmacology 112:221–227. https://doi.org/10.1016/j.neuropharm.2016.08.022
French JA, Krauss GL, Steinhoff BJ, SquillacoteD YangH, Kumar D et al (2013) Evaluation of adjunctive perampanel in patients with refractory partial-onset seizures: results of randomized global phase III study 305. Epilepsia 54:1117–1125. https://doi.org/10.1111/j.1528-1167.2012.03638.x
Krauss GL, Serratosa JM, Villanueva V, Endziniene M, Hong Z, French J (2012) Randomized phase III study 306: adjunctive perampanel for refractory partial-onset seizures. Neurology 78:1408–1415. https://doi.org/10.1212/WNL.0b013e318254473a
Dohare P, Zia MT, Ahmed E, Asad A, Yadala V, Schober AL et al (2016) AMPA-kainate receptor inhibition promotes neurologic recovery in premature rabbits with intraventricular hemorrhage. J Neurosci 36:3363–3377. https://doi.org/10.1523/JNEUROSCI.4329-15.201
Wu T, Ido K, Osada Y, Kotani S, Tamaoka A, Hanada T (2017) The neuroprotective effect of perampanel in lithium-pilocarpine rat seizure model. Epilepsy Res 137:152–158. https://doi.org/10.1016/j.eplepsyres.2017.06.002
Huston JP, Chao OY (2023) Probing the nature of episodic memory in rodents. Neurosci Biobehav Rev 144:104930. https://doi.org/10.1016/j.neubiorev.2022.104930
Izquierdo I, Furini CR, Myskiw JC (2016) Fear memory. Physiol Rev 96(2):695–750. https://doi.org/10.1152/physrev.00018.2015
Cassaday HJ, Muir C, Stevenson CW, Bonardi C, Hock R, Waite L (2023) From safety to frustration: the neural substrates of inhibitory learning in aversive and appetitive conditioning procedures. Neurobiol Learn Mem 202:107757. https://doi.org/10.1016/j.nlm.2023.107757
Zolkowska D, Dhir A, Rogawski MA (2021) Perampanel, a potent AMPA receptor antagonist, protects against tetramethylenedisulfotetramine-induced seizures and lethality in mice: comparison with diazepam. Arch Toxicol 95(7):2459–2468. https://doi.org/10.1016/j.eplepsyres.2017.06.002
Alqahtani F, Assiri MA, Mohany M, Imran I, Javaid S, Rasool MF et al (2020) Coadministration of ketamine and perampanel improves behavioral function and reduces inflammation in acute traumatic brain injury mouse model. Biomed Res 10(2020):3193725. https://doi.org/10.1155/2020/3193725
de Lima MN, Luft T, Roesler R, Schröder N (2006) Temporary inactivation reveals an essential role of the dorsal hippocampus in consolidation of object recognition memory. Neurosci Lett 405(1–2):142–146. https://doi.org/10.1016/j.neulet.2006.06.044
De Souza LO, Machado GDB, de Freitas BS, Rodrigues SLC, Severo MPA, Molz P et al (2021) The G protein-coupled estrogen receptor (GPER) regulates recognition and aversively-motivated memory in male rats. Neurobiol Learn Mem 184:107499. https://doi.org/10.1016/j.nlm.2021.107499
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
da Silva VK, de Freitas BS, Garcia RCL, Monteiro RT, Hallak JE, Zuardi AW et al (2018) Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl Psychiatry 8(1):176. https://doi.org/10.1038/s41398-018-0232-5
Molz P, de Freitas BS, Uberti VH, da Costa KM, Kist LW, Bogo MR et al (2021) Effects of lipoic acid supplementation on age- and iron-induced memory impairment, mitochondrial DNA damage and antioxidant responses. Eur J Nutr 60(7):3679–3690. https://doi.org/10.1007/s00394-021-02541-z
Mohammad H, Sekar S, Wei Z, Moien-Afshari F, Taghibiglou C (2019) Perampanel but not amantadine prevents behavioral alterations and epileptogenesis in pilocarpine rat model of status epilepticus. Mol Neurobiol 56(4):2508–2523. https://doi.org/10.1007/s12035-018-1230-6
da Silva VK, de Freitas BS, Dornelles VC, Kist LW, Bogo MR, Silva MC, Streck EL, Hallak JE et al (2018) Novel insights into mitochondrial molecular targets of iron-induced neurodegeneration: reversal by cannabidiol. Brain Res Bull 139:1–8. https://doi.org/10.1016/j.brainresbull.2018.01.014
Bellingacci L, Tallarico M, Mancini A, Megaro A, De Caro C, Citraro R et al (2023) Non-competitive AMPA glutamate receptors antagonism by perampanel as a strategy to counteract hippocampal hyper-excitability and cognitive deficits in cerebral amyloidosis. Neuropharmacology 225:109373. https://doi.org/10.1016/j.neuropharm.2022.109373
Chen T, Dai SH, Jiang ZQ, Luo P, Jiang XF, Fei Z et al (2017) The AMPAR antagonist perampanel attenuates traumatic brain injury through anti-oxidative and anti-inflammatory activity. Cell Mol Neurobiol 37(1):43–52. https://doi.org/10.1007/s10571-016-0341-8
Carota G, Distefano A, Spampinato M, Giallongo C, Broggi G, Longhitano L et al (2022) Neuroprotective Role of α-lipoic acid in iron-overload-mediated toxicity and inflammation in in vitro and in vivo model. Antioxidants (Basel) 11(8):1596. https://doi.org/10.3390/antiox11081596
Di Filippo M, Mancini A, Bellingacci L, Gaetani L, Mazzocchetti P, Zelante T et al (2021) Interleukin-17 affects synaptic plasticity and cognition in an experimental model of multiple sclerosis. Cell Rep 37(10):110094. https://doi.org/10.1016/j.celrep.2021.110094
Centonze D, Muzio L, Rossi S, Cavasinni F, De Chiara V, Bergami A et al (2009) Inflammation triggers synaptic alteration and degeneration in experimental autoimmune encephalomyelitis. J Neurosci 29:3442–3452. https://doi.org/10.1523/JNEUROSCI.5804-08.2009
Rogawski MA, Hanada T (2013) Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl 197:19–24. https://doi.org/10.1111/ane.12100
Takahashi T (2011) Mechanisms underlying contextual fear learning. Commun Integr Biol 4(6):726–727. https://doi.org/10.4161/cib.17505
Marcondes LA, Myskiw J de C, Nachtigall EG, Narvaes RF, Izquierdo I, Furini CRG (2022) PKMζ maintains remote contextual fear memory by inhibiting GluA2-dependent AMPA receptor endocytosis in the prelimbic cortex. Neuroscience 497:97–106. https://doi.org/10.1016/j.neuroscience.2021.12.028
Collingridge GL, Olsen RW, Peters J, Spedding M (2009) A nomenclature for ligand-gated ion channels. Neuropharmacology 56:2–5. https://doi.org/10.1016/j.neuropharm.2008.06.063
Twomey EC, Yelshanskaya MV, Grassucci RA, Frank J, Sobolevsky AI (2017) Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nature 549(7670):60–65. https://doi.org/10.1038/nature23479
Wright A, Vissel B (2012) The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front Mol Neurosci 5:34. https://doi.org/10.3389/fnmol.2012.00034
Han M, Kim J (2015) Effect of dietary iron loading on recognition memory in growing rats. PLoS ONE 10(3):e0120609. https://doi.org/10.1371/journal.pone.0120609
Brown JC, Higgins ES, George MS (2022) Synaptic plasticity 101: the story of the AMPA receptor for the brain stimulation practitioner. Neuromodulation 25(8):1289–1298. https://doi.org/10.1016/j.neurom.2021.09.003
An JR, Su JN, Sun GY et al (2022) Liraglutide alleviates cognitive deficit in db/db mice: involvement in oxidative stress, iron overload, and ferroptosis. Neurochem Res 47(2):279–294. https://doi.org/10.1007/s11064-021-03442-7
Ghobadi M, Akbari S, Bayat M, Moosavi SMS, Salehi MS, Pandamooz S, Azarpira N, Afshari A et al (2024) Gens PSD-95 and GSK-3beta expression improved by hair follicular stem cells-conditioned medium enhances synaptic transmission and cognitive abilities in the rat model of vascular dementia. Brain Behav 14(1):e3351. https://doi.org/10.1002/brb3.3351
Wang M, Feng N, Qin J, Wang S, Chen J, Qian S, Liu Y, Luo F (2024) Abdominal surgery under ketamine anesthesia during second trimester impairs hippocampal learning and memory of offspring by regulating dendrite spine remodeling in rats. Neurotoxicology 101:82–92. https://doi.org/10.1016/j.neuro.2024.02.003
van der Spek SJF, Pandya NJ, Koopmans F (2022) Expression and interaction proteomics of GluA1- and GluA3-subunit-containing AMPARs reveal distinct protein composition. Cells 11(22):3648. https://doi.org/10.3390/cells11223648
Yang W, Ma L, Hai DM, Liu N, Yang JM, Lan XB et al (2022) Hippocampal proteomic analysis in male mice following aggressive behavior induced by long-term administration of perampanel. ACS Omega 7(23):19388–19400. https://doi.org/10.1021/acsomega.2c01008
Kim JE, Lee DS, Park H, Kim TH, Kang TC (2021) Inhibition of AKT/GSK3β/CREB pathway improves the responsiveness to AMPA receptor antagonists by regulating GRIA1 surface expression in chronic Epilepsy rats. Biomedicines 9(4):425. https://doi.org/10.3390/biomedicines9040425
Kim JE, Park H, Lee JE, Kim TH, Kang TC (2020) PTEN is required for the anti-epileptic effects of AMPA receptor antagonists in chronic epileptic rats. Int J Mol Sci 21(16):5643. https://doi.org/10.3390/ijms21165643
Kim JE, Choi HC, Song HK, Kang TC (2019) Perampanel affects up-stream regulatory signaling pathways of GluA1 phosphorylation in normal and epileptic rats. Front Cell Neurosci 13:80. https://doi.org/10.3389/fncel.2019.00080
Kondo M, Sumino R, Okado H (2000) Expression of AMPA receptors in rat superior colliculus and effect of orbital enucleation. Brain Res 883(2):238–242. https://doi.org/10.1016/s0006-8993(00)02879-1
Whitcomb DJ, Hogg EL, Regan P, Piers T, Narayan P, Whitehead G et al (2015) Intracellular oligomeric amyloid-beta rapidly regulates GluA1 subunit of AMPA receptor in the hippocampus. Sci Rep 5:10934. https://doi.org/10.1038/srep10934
Stafford J, Brownlow ML, Qualley A, Jankord R (2018) AMPA receptor translocation and phosphorylation are induced by transcranial direct current stimulation in rats. Neurobiol Learn Mem 150:36–41. https://doi.org/10.1016/j.nlm.2017.11.002
Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M (2005) Memory consolidation induces N-methyl-D-aspartic acid-receptor- and Ca2+/calmodulin-dependent protein kinase II-dependent modifications in alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor properties. Neuroscience 136(2):397–403. https://doi.org/10.1016/j.neuroscience.2005.08.007
Sathler MF, Khatri L, Roberts JP, Schmidt IG, Zaytseva A, Kubrusly RCC et al (2021) Phosphorylation of the AMPA receptor subunit GluA1 regulates clathrin-mediated receptor internalization. J Cell Sci 134(17):jcs257972. https://doi.org/10.1242/jcs.257972
Ji SG, Weiss JH (2018) Zn2+-induced disruption of neuronal mitochondrial function: synergism with Ca2+, critical dependence upon cytosolic Zn2+ buffering, and contributions to neuronal injury. Exp Neurol 302:181–195. https://doi.org/10.1016/j.expneurol.2018.01.012
Baracaldo-Santamaría D, Avendaño-Lopez SS, Ariza-Salamanca DF, Rodriguez-Giraldo M, Calderon-Ospina CA, González-Reyes RE, Nava-Mesa MO (2023) Role of calcium modulation in the pathophysiology and treatment of Alzheimer’s disease. Int J Mol Sci 24(10):9067. https://doi.org/10.3390/ijms24109067
Funding
This work was supported by National Council for Scientific and Technological Development [CNPq; grant numbers 403154/2021–9 and 305656/2019–8 to N.S.]; the National Institute of Science and Technology for Translational Medicine [INCT-TM—grant number 465458/2014–9]; and Rio Grande do Sul State Research Foundation (FAPERGS—grant number 22/2551–0000385-0); N.S. is Research Career Awardee of the CNPq. The funding sources were not involved in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
Conceptualization: J da Silva, N Schroder; methodology: J da Silva and N Schroder; formal analysis and investigation: J da Silva, LO de Souza, MPA Severo, SLC Rodrigues, P Molz, P Schonhofen, and AL Herlinger; writing—original draft preparation: J da Silva; writing—review and editing: N Schroder; funding acquisition: N Schroder; resources: MPA Severo, S L C Rodrigues, and P Molz; supervision: N Schroder.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics Approval
This study was approved by the Institutional Ethics Committee for the Use of Animals of the Federal University of Rio Grande do Sul (CEUA, #35604), and all experimental procedures were performed in accordance with the Brazilian Guidelines for the Care and Use of Animals in Research and Teaching (DBCA, published by CONCEA, MCTI, Brazil).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Silva, J., de Souza, L.O., Severo, M.P.A. et al. Effects of the AMPAR Antagonist, Perampanel, on Cognitive Function in Rats Exposed to Neonatal Iron Overload. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-024-04180-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-024-04180-x