Abstract
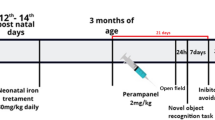
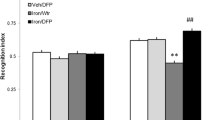
Alterations of brain iron levels have been observed in a number of neurodegenerative disorders. We have previously demonstrated that iron overload in the neonatal period results in severe and persistent memory deficits in the adulthood. Protein degradation mediated by the ubiquitin-proteasome system (UPS) plays a central regulatory role in several cellular processes. Impairment of the UPS has been implicated in the pathogenesis of neurodegenerative disorders. Here, we examined the effects of iron exposure in the neonatal period (12th–14th day of postnatal life) on the expression of proteasome β-1, β-2, and β-5 subunits, and ubiquitinated proteins in brains of 15-day-old rats, to evaluate the immediate effect of the treatment, and in adulthood to assess long-lasting effects. Two different memory types, emotionally motivated conditioning and object recognition were assessed in adult animals. We found that iron administered in the neonatal period impairs both emotionally motivated and recognition memory. Polyubiquitinated protein levels were increased in the hippocampus, but not in the cortex, of adult animals treated with iron. Gene expression of subunits β1 and β5 was affected by age, being higher in the early stages of development in the hippocampus, accompanied by an age-related increase in polyubiquitinated protein levels in adults. In the cortex, gene expression of the three proteasome subunits was significantly higher in adulthood than in the neonatal period. These findings suggest that expression of proteasome subunits and activity are age-dependently regulated. Iron exposure in the neonatal period produces long-lasting harmful effects on the UPS functioning, which may be related with iron-induced memory impairment.






Similar content being viewed by others
References
Wong E, Cuervo AM (2011) Integration of clearance mechanisms: the proteasome and autophagy. Cold Spring Harb Perspect Biol 2:a006734
Ciechanover A, Brundin P (2003) The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40:427–446
Heinemeyer W, Fischer M, Krimmer T, Stachon U, Wolf DH (1997) The active sites of the eukaryotic 20 S proteasome and their involvement in subunit precursor processing. J Biol Chem 272:25200–25209
Voges D, Zwickl P, Baumeister W (1999) The 26 S proteasome: a molecular machine designed for controlled proteolysis. Annu Rev Biochem 68:1015–1068
Bochtler M, Ditzel L, Groll M, Hartmann C, Huber R (1999) The proteasome. Annu Rev Biophys Biomol Struct 28:295–317
Hershko A, Ciechanover A (1998) The ubiquitin system. Annu Rev Biochem 67:425–479
Yao X, Liu J, McCabe JT (2008) Alterations of cerebral cortex and hippocampal proteasome subunit expression and function in a traumatic brain injury rat model. J Neurochem 104:353–363
Jadhav T, Wooten MW (2009) Defining an embedded code for protein ubiquitination. J Proteomics Bioinform 2:316–333
Hegde AN, Upadhya SC (2011) Role of ubiquitin-proteasome-mediated proteolysis in nervous system disease. Biochim Biophys Acta 1809:128–140
Keck S, Nitsch R, Grune T, Ullrich O (2003) Proteasome inhibition by paired helical filament-tau in brains of patients with Alzheimer’s disease. J Neurochem 85:115–122
Keller JN, Hanni KB, Markesbery WR (2000) Impaired proteasome function in Alzheimer’s disease. J Neurochem 75:436–439
Lopez-Salon M, Morelli L, Castano EM, Soto EF, Pasquini JM (2000) Defective ubiquitination of cerebral proteins in Alzheimer’s disease. J Neurosci Res 62:302–310
Gavilán MP, Vela J, Castaño A, Ramos B, del Río JC, Vitorica J, Ruano D (2006) Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging 27:973–982
Keller JN, Hanni KB, Markesbery WR (2000) Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev 113:61–70
Bedford L, Hay D, Devoy A et al (2008) Depletion of 26S proteasomes in mouse brain neurons causes neurodegeneration and Lewy-like inclusions resembling human pale bodies. J Neurosci 28:8189–8198
Betarbet R, Canet-Aviles RM, Sherer TB et al (2006) Intersecting pathways to neurodegeneration in Parkinson’s disease: Effects of the pesticide rotenone on DJ-1, α-synuclein, and the ubiquitin-proteasome system. Neurobiol of Dis 22:404–420
Seo H, Sonntag KC, Kim W, Cattaneo E, Isacson O (2007) Proteasome activator enhances survival of Huntington’s disease neuronal model cells. PloS ONE 2(2), e238
Schipper HM (2012) Neurodegeneration with brain iron accumulation—clinical syndromes and neuroimaging. Biochim Biophys Acta 1822:350–360
Ward RJ, Zucca FA, Duyn JH, Crichton RR, Zecca L (2014) The role of iron brain ageing and neurodegenerative disorders. Lancet Neurol 13:1045–1060
Zecca L, Youdim MB, Riederer P, Connor JR, Crichton RR (2004) Iron, brain ageing and neurodegenerative disorders. Nat Rev Neurosci 5(11):863–873
Kell DB (2010) Towards a unifying, systems biology understanding of large-scale cellular death and destruction caused by poorly liganded iron: Parkinson’s, Huntington’s, prions, bactericides, chemical toxicology and others as examples. Arch Toxicol 84:825–889
Schröder N, Figueiredo L, de Lima MN (2013) Role of brain iron accumulation in cognitive dysfunction: evidence from animal models and human studies. J Alzheimers Dis 34:797–812
Schröder N, Fredriksson A, Vianna MR, Roesler R, Izquierdo I, Archer T (2001) Memory deficits in adult rats following post natal iron administration. Behav Brain Res 124(1):77–85
De Lima MN, Polydoro M, Laranja DC, Bonatto F, Bromberg E, Moreira JC, Dal-Pizzol F, Schröder N (2005) Recognition memory impairment and brain oxidative stress by postnatal iron administration. Eur J Neurosci 21:2521–2528
Da Silva VK, De Freitas BS, Da Silva AD et al (2014) Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: implications for neuroprotection. Mol Neurobiol 49:222–233
Budni P, de Lima MN, Polydoro M, Moreira JC, Schröder N, Dal-Pizzol F (2007) Antioxidant effects of selegiline in oxidative stress induced by iron neonatal treatment in rats. Neurochem Res 32:965–972
Dal-Pizzol F, Klamt F, Frota ML et al (2001) Neonatal iron exposure induces oxidative stress in adult Wistar rat. Brain Res Dev 130:109–114
Miwa CP, de Lima MN, Scalco F et al (2011) Neonatal iron treatment increases apoptotic markers in hippocampal and cortical areas of adult rats. Neurotox Res 19:527–535
Fernandez LL, de Lima MN, Scalco F, Vedana G, Miwa C, Hilbig A, Vianna MR, Schröder N (2011) Early post-natal iron administration induces astroglial response in the brain of adult and aged rats. Neurotox Res 20:193–199
Fagherazzi EV, Garcia VA, Maurmann N et al (2011) Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology 219(4):1133–1140
Silva PF, Garcia VA, Da Dornelles AS et al (2012) Memory impairment induced by brain iron overload is accompanied by reduced H3K9 acetylation and ameliorated by sodium butyrate. Neuroscience 200:42–49
Taylor EM, Morgan EH (1990) Developmental changes in transferrin and iron uptake by the brain in the rat. Brain Res Dev Brain Res 55:35–42
Taylor EM, Crowe A, Morgan EH (1991) Transferrin and iron uptake by the brain: effects of altered iron status. J Neurochem 57:1584–1592
Izquierdo I, Medina JH (1997) Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain structures. Neurobiol Learn Mem 68:285–316
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Pilchova I, Klacanova K, Chomova M, Tatarkova Z, Dobrota D, Racay P (2015) Possible contribution of proteins of Bcl-2 family in neuronal death following transient global brain ischemia. Cell Mol Neurobiol 35:23–31
Bhat KP, Yan S, Wang CE, Li S, Li XJ (2014) Differential ubiquitination and degradation of huntingtin fragments modulated by ubiquitin-protein ligase E3A. Proc Natl Acad Sci U S A 111:5706–5711
Bonefeld BE, Elfving B, Wegener G (2008) Reference genes for normalization: a study of rat brain tissue. Synapse 62:302–309
Bustin SA, Benes V, Garson J et al (2013) The need for transparency and good practices in the qPCR literature. Nat Methods 10:1063–1067
Rodrigue KM, Daugherty AM, Haacke EM, Raz N (2013) The role of hippocampal volume in age-related differences in memory. Cereb Cortex 23(7):1533–1541
Figueiredo LS, Dornelles AS, Petry FS et al (2015) Two waves of proteasome-dependent protein degradation in the hippocampus are required for recognition memory consolidation. Neurobiol Learn Mem 120:1–6
Hegde AN, Inokuchi K, Pei W et al (1997) Ubiquitin C-terminal hydrolase is an immediate-early gene essential for long-term facilitation in aplysia. Cell 89:115–126
Jarome TJ, Werner CT, Kwapis JL, Helmstetter FJ (2011) Activity dependent protein degradation is critical for the formation and stability of fear memory in the amygdale. PLoS ONE 6:e24349
Reis DS, Jarome TJ, Helmstetter FJ (2013) Memory formation for trace fear conditioning requires ubiquitin-proteasome mediated degradation in the prefrontal cortex. Front Behav Neurosci 7:1–7
Lopez-Salon M, Alonso M, Vianna MR, Viola H, Mello e Souza T, Izquierdo I, Pasquini JM, Medina JH (2001) The ubiquitin-proteasome cascade is required for mammalian long-term memory formation. Eur J Neurosci 14:1820–1826
Zhu W, Li X, Xie W, Luo F, Kaur D, Andersen JK, Jankovic J, Le W (2010) Genetic iron chelation protects against proteosome inhibition-induced dopamine neuron degeneration. Neurobiol Dis 37:307–313
Gavilán MP, Pintado C, Gavilán E, Jiménez S, Ríos RM, Vitorica J, Castaño A, Ruano D (2009) Dysfunction of the unfolded protein response increases neurodegeneration in aged rat hippocampus following proteasome inhibition. Aging Cell 8:654–665
Pintado C, Gavilán M, Gavilán E et al (2012) Lipopolysaccharide-induced neuroinflammation leads to the accumulation of ubiquitinated proteins and increases susceptibility to neurodegeneration induced by proteasome inhibition in rat hippocampus. J Neuroinflammation 9:87
Nunziante M, Ackermann K, Dietrich K et al (2011) Proteasomal dysfunction and endoplasmic reticulum stress enhance trafficking of prion protein aggregates through the secretory pathway and increase accumulation of pathologic prion protein. J Biol Chem 286:33942–33953
Hare DJ, Arora M, Jenkins NL, Finkelstein DI, Doble PA, Bush AI (2015) Is early-life iron exposure critical in neurodegeneration? Nat Rev Neurol 11:536–544
Keller JN, Dimayuga E, Chen Q, Thorpe J, Gee J, Ding Q (2004) Autophagy, proteasomes, lipofuscin, and oxidative stress in the aging brain. Int J Biochem Cell Biol 36:2376–2391
Zhu W, Xie W, Pan T et al (2007) Prevention and restoration of lactacystin-induced nigrostriatal dopamine neuron degeneration by novel brain-permeable iron chelators. FASEB J 21:3835–3844
Martinez-Vicente M, Sovak G, Cuervo AN (2005) Protein degradation and aging. Exp Gerontol 40:622–633
Giannini C, Kloβ A, Gohlke S, Mishto M, Nicholson TP, Sheppard PW, Kloetzel PM, Dahlmann B (2013) Poly-Ub-substrate-degradative activity of 26S proteasome is not impairment in the aging rat brain. Plos One 8(5):e64042
Acknowledgments
This research was supported by the National Council for Scientific and Technological Development (CNPq). L.S.F. is supported by a CAPES/MEC fellowship. V.A.D. is supported by a CNPq/PIBIC scholarship and L.M.K. is supported by a BPA/PUCRS scholarship. L.W.K. is recipient of fellowship CAPES/PNPD Program. M.R.B. and N.S. are Research Career Awardees of the CNPq.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Figueiredo, L.S., de Freitas, B.S., Garcia, V.A. et al. Iron Loading Selectively Increases Hippocampal Levels of Ubiquitinated Proteins and Impairs Hippocampus-Dependent Memory. Mol Neurobiol 53, 6228–6239 (2016). https://doi.org/10.1007/s12035-015-9514-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-015-9514-6