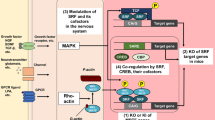
Abstract
Glioblastomas derived from malignant astrocytes are the most common primary tumors of the central nervous system in humans, exhibiting very bad prognosis. Treatment with surgery, radiotherapy, and chemotherapy (mainly using temozolomide), generates as much one-year survival. The circadian clock controls different aspects of tumor development, and its role in GBM is beginning to be explored. Here, the role of the canonic circadian clock gene bmal1 was studied in vivo in a nude mice model bearing human GBMs from LN229 cells xenografted orthotopically in the dorsal striatum. For that aim, a bmal1 knock-down was generated in LN229 cells by CRISPR/Cas9 gene editing tool, and tumor progression was followed in male mice by measuring survival, tumor growth, cell proliferation and prognosis with CD44 marker, as well as astrocyte activation in the tumor microenvironment with GFAP and nestin markers. Disruption of bmal1 in the tumor decreased survival, increased tumor growth and CD44 expression, worsened motor performance, as well as increased GFAP expression in astrocytes at tumor microenvironment. In addition, survival and tumor progression was not affected in mice bearing LN229 wild type GBM that underwent circadian disruption by constant light, as compared to mice synchronized to 12:12 light–dark cycles. These results consistently demonstrate in an in vivo orthotopic model of human GBM, that bmal1 has a key role as a tumor suppressor gene regulating GBM progression.
Graphical Abstract










Similar content being viewed by others
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to Laboratory Policies, but are available from the corresponding author on reasonable request.
References
Omuro A, De Angelis LM (2013) Glioblastoma and other malignant gliomas: a clinical review. JAMA - J Am Med Assoc. https://doi.org/10.1001/jama.2013.280319
Phillips HS et al (2006) Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell. https://doi.org/10.1016/j.ccr.2006.02.019
Stupp R et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Golombek DA, Rosenstein RE (2010) Physiology of Circadian Entrainment. Physiol Rev. https://doi.org/10.1152/physrev.00009.2009
Hastings MH, Maywood ES, Brancaccio M (2018) Generation of circadian rhythms in the suprachiasmatic nucleus. Nat Rev Neurosci 19(8):453–469. https://doi.org/10.1038/s41583-018-0026-z
Takahashi JS (2015) Molecular components of the circadian clock in mammals. Diabetes Obes Metab 17(Suppl 1):6–11. https://doi.org/10.1111/dom.12514
Bunger MK et al (2000) Mop3 is an essential component of the master circadian pacemaker in mammals. Cell 103(7):1009–1017. https://doi.org/10.1016/s0092-8674(00)00205-1
Zhao C et al (2022) Circadian clock gene BMAL1 inhibits the proliferation and tumor-formation ability of nasopharyngeal carcinoma cells and increases the sensitivity of radiotherapy. Chronobiol Int 39(10):1340–1351. https://doi.org/10.1080/07420528.2022.2105708
Jiang W et al (2016) The circadian clock gene Bmal1 acts as a potential anti-oncogene in pancreatic cancer by activating the p53 tumor suppressor pathway. Cancer Lett 371(2):314–325. https://doi.org/10.1016/j.canlet.2015.12.002
Qu M et al (2023) Circadian regulator BMAL1: CLOCK promotes cell proliferation in hepatocellular carcinoma by controlling apoptosis and cell cycle. Proc Natl Acad Sci U S A 120(2):e2214829120. https://doi.org/10.1073/pnas.2214829120
Wang J et al (2019) Circadian protein BMAL1 promotes breast cancer cell invasion and metastasis by up-regulating matrix metalloproteinase9 expression. Cancer Cell Int 19:182. https://doi.org/10.1186/s12935-019-0902-2
Dong P et al (2022) BMAL1 induces colorectal cancer metastasis by stimulating exosome secretion. Mol Biol Rep 49(1):373–384. https://doi.org/10.1007/s11033-021-06883-z
Wang D et al (2023) Identification and characterization of the CDK1-BMAL1-UHRF1 pathway driving tumor progression. iScience 26(4):106544. https://doi.org/10.1016/j.isci.2023.106544
Masri S, Cervantes M, Sassone-Corsi P (2013) The circadian clock and cell cycle: interconnected biological circuits. Curr Opin Cell Biol 25(6):730–734. https://doi.org/10.1016/j.ceb.2013.07.013
Fu XJ et al (2016) The important tumor suppressor role of PER1 in regulating the cyclin–CDK–CKI network in SCC15 human oral squamous cell carcinoma cells. Onco Targets Ther https://doi.org/10.2147/OTT.S100952
Gery S et al (2006) The circadian gene per1 plays an important role in cell growth and DNA damage control in human cancer cells. Mol Cell 22(3):375–382
Chen ST et al (2005) Deregulated expression of the PER1, PER2 and PER3 genes in breast cancers. Carcinogenesis https://doi.org/10.1093/carcin/bgi075
Zhao H et al (2014) Prognostic relevance of Period1 (Per1) and Period2 (Per2) expression in human gastric cancer. Int J Clin Exp Pathol 7(2):619–30
Hsu CM et al (2012) Altered expression of circadian clock genes in head and neck squamous cell carcinoma. Tumor Biol 33(1):149–55. https://doi.org/10.1007/s13277-011-0258-2
Farshadi E et al (2019) The positive circadian regulators CLOCK and BMAL1 control G2/M cell cycle transition through Cyclin B1. Cell Cycle 18(1):16–33. https://doi.org/10.1080/15384101.2018.1558638
Matsuo T et al (2003) Control mechanism of the circadian clock for timing of cell division in vivo. Science 302(5643):255–9. https://doi.org/10.1126/science.1086271
Gwon DH et al (2020) BMAL1 suppresses proliferation, migration, and invasion of U87MG cells by downregulating cyclin B1, Phospho-AKT, and Metalloproteinase-9. Int J Mol Sci 21(7):2352. https://doi.org/10.3390/ijms21072352
Jung CH et al (2013) Bmal1 suppresses cancer cell invasion by blocking the phosphoinositide 3-kinase-Akt-MMP-2 signaling pathway. Oncol Rep 29(6):2109–2113. https://doi.org/10.3892/or.2013.2381
Wagner PM et al (2021) Temporal regulation of tumor growth in nocturnal mammals: in vivo studies and chemotherapeutical potential. FASEB J 35(2):e21231. https://doi.org/10.1096/fj.202001753R
Dong Z et al (2019) Targeting glioblastoma stem cells through disruption of the circadian clock. Cancer Discov 9(11):1556–1573. https://doi.org/10.1158/2159-8290.CD-19-0215
Pang L et al (2023) Circadian regulator CLOCK promotes tumor angiogenesis in glioblastoma. Cell Rep 42(2):112127. https://doi.org/10.1016/j.celrep.2023.112127
Xuan W et al (2022) Circadian Regulator CLOCK Drives Immunosuppression in Glioblastoma. Cancer Immunol Res 10(6):770–784. https://doi.org/10.1158/2326-6066.CIR-21-0559
Filipski E et al (2004) Effects of chronic jet lag on tumor progression in mice. Cancer Res 64(21):7879–7885
Aiello I et al (2020) Circadian disruption promotes tumor-immune microenvironment remodeling favoring tumor cell proliferation. Sci Adv. 6(42):eaaz4530. https://doi.org/10.1126/sciadv
Hadadi E et al (2020) Chronic circadian disruption modulates breast cancer stemness and immune microenvironment to drive metastasis in mice. Nat Commun 11(1):3193. https://doi.org/10.1038/s41467-020-16890-6
Wagner PM et al (2019) Proliferative glioblastoma cancer cells exhibit persisting temporal control of metabolism and display differential temporal drug susceptibility in chemotherapy. Mol Neurobiol 56(2):1276–1292. https://doi.org/10.1007/s12035-018-1152-3
Trebucq LL et al (2021) Timing of novel Drug 1A–116 to circadian rhythms improves therapeutic effects against glioblastoma. Pharmaceutics 13(7):1091. https://doi.org/10.3390/pharmaceutics13071091
Montgomery CA Jr (1990) Oncological and toxicological research: Alleviation and control of pain and distress in laboratory animals. Cancer Bulletin 42:230–237
Magno LAV, Collodetti M, Tenza-Ferrer H, Romano-Silva MA (2019) Cylinder test to assess sensory-motor function in a mouse model of Parkinson’s disease. Bio Protoc 9(16):e3337. https://doi.org/10.21769/BioProtoc.3337
King GD et al (2008) Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther 16(4):682–690. https://doi.org/10.1038/mt.2008.18
Wu G et al (2016) MetaCycle: an integrated R package to evaluate periodicity in large scale data. Bioinformatics. https://doi.org/10.1093/bioinformatics/btw405
El-Athman R, Fuhr L, Relógio A (2018) A systems-level analysis reveals circadian regulation of splicing in colorectal cancer. EBioMedicine 33:68–81. https://doi.org/10.1016/j.ebiom.2018.06.012
Xiang R et al (2018) Circadian clock gene Per2 downregulation in non-small cell lung cancer is associated with tumor progression and metastasis. Oncol Rep 40(5):3040–3048. https://doi.org/10.3892/or.2018.6704
Mooney KL et al (2016) The role of CD44 in glioblastoma multiforme. J Clin Neurosci 34:1–5. https://doi.org/10.1016/j.jocn.2016.05.012
Yu M, Li W, Wang Q, Wang Y, Lu F (2018) Circadian regulator NR1D2 regulates glioblastoma cell proliferation and motility. Oncogene 37(35):4838–4853. https://doi.org/10.1038/s41388-018-0319-8
Dzobo K et al (2020) Advances in therapeutic targeting of cancer stem cells within the tumor microenvironment: an updated review. Cells 9(8):1896. https://doi.org/10.3390/cells9081896
Hou C et al (2019) Overexpression of CD44 is associated with a poor prognosis in grade II/III gliomas. J Neurooncol 145(2):201–210. https://doi.org/10.1007/s11060-019-03288-8
Ameneiro C et al (2020) BMAL1 coordinates energy metabolism and differentiation of pluripotent stem cells. Life Sci Alliance 3(5):e201900534. https://doi.org/10.26508/lsa.201900534
Kim Y, Lee YS, Choe J, Lee H, Kim YM, Jeoung D (2008) CD44-epidermal growth factor receptor interaction mediates hyaluronic acid-promoted cell motility by activating protein kinase C signaling involving Akt, Rac1, Phox, reactive oxygen species, focal adhesion kinase, and MMP-2. J Biol Chem 283(33):22513–28. https://doi.org/10.1074/jbc.M708319200
Li Z et al (2017) The OncoPPi network of cancer-focused protein-protein interactions to inform biological insights and therapeutic strategies. Nat Commun 8:14356. https://doi.org/10.1038/ncomms14356
Chen P et al (2020) Circadian regulator CLOCK recruits immune-suppressive microglia into the GBM tumor microenvironment. Cancer Discov 10(3):371–381. https://doi.org/10.1158/2159-8290.CD-19-0400
Wang F, Li C, Han F, Chen L, Zhu L (2021) BMAL1 may be involved in angiogenesis and peritumoral cerebral edema of human glioma by regulating VEGF and ANG2. Aging (Albany NY) 13(22):24675–24685. https://doi.org/10.18632/aging.203708
Matias D et al (2018) Microglia/astrocytes-glioblastoma crosstalk: crucial molecular mechanisms and microenvironmental factors. Front Cell Neurosci 12:235. https://doi.org/10.3389/fncel.2018.00235
O’Brien ER, Howarth C, Sibson NR (2013) The role of astrocytes in CNS tumors: pre-clinical models and novel imaging approaches. Front Cell Neurosci 7:40. https://doi.org/10.3389/fncel.2013.00040
Escartin C et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci 24(3):312–325. https://doi.org/10.1038/s41593-020-00783-4
Du Z et al (2022) Association of glioma CD44 expression with glial dynamics in the tumour microenvironment and patient prognosis. Comput Struct Biotechnol J 20:5203–5217. https://doi.org/10.1016/j.csbj.2022.09.003
Maroni MJ et al (2018) Constant light alters serum hormone levels related to thyroid function in male CD-1 mice. Chronobiol Int 35(10):1456–1463. https://doi.org/10.1080/07420528.2018.1488259
Rumanova VS, Okuliarova M, Zeman M (2020) Differential effects of constant light and dim light at night on the circadian control of metabolism and behavior. Int J Mol Sci 21(15):5478. https://doi.org/10.3390/ijms21155478
Duhart JM, Brocardo L, Caldart CS, Marpegan L, Golombek DA (2017) Circadian alterations in a murine model of hypothalamic glioma. Front Physiol 8:864. https://doi.org/10.3389/fphys.2017.00864
Abdraboh ME et al (2022) Constant light exposure and/or pinealectomy increases susceptibility to trichloroethylene-induced hepatotoxicity and liver cancer in male mice. Environ Sci Pollut Res Int 29(40):60371–60384. https://doi.org/10.1007/s11356-022-19976-4
Anisimov VN et al (2012) Light-at-night-induced circadian disruption, cancer, and aging. Curr Aging Sci 5(3):170–177. https://doi.org/10.2174/1874609811205030002
Khan S et al (2019) Impact of chronically alternating light-dark cycles on circadian clock mediated expression of cancer (glioma)-related genes in the brain. Int J Biol Sci 15(9):1816–1834. https://doi.org/10.7150/ijbs.35520
Khan S, Yong VW, Xue M (2021) Circadian disruption in mice through chronic jet lag-like conditions modulates molecular profiles of cancer in nucleus accumbens and prefrontal cortex. Carcinogenesis 42(6):864–873. https://doi.org/10.1093/carcin/bgab012
Guerrero-Vargas NN et al (2017) Circadian disruption promotes tumor growth by anabolic host metabolism, experimental evidence in a rat model. BMC Cancer 17(1):625. https://doi.org/10.1186/s12885-017-3636-3
Ohta H et al (2005) Constant light desynchronizes mammalian clock neurons. Nat Neurosci 8(3):267–269. https://doi.org/10.1038/nn1395
Wagner PM et al (2021) Adjusting the molecular clock: the importance of circadian rhythms in the development of glioblastomas and its intervention as a therapeutic strategy. 22(15):8289. https://doi.org/10.3390/ijms22158289
Cederroth CR et al (2019) Medicine in the fourth dimension. Cell Metab 30(2):238–250. https://doi.org/10.1016/j.cmet.2019.06.019
Acknowledgements
Special thanks to Dr. Santiago Plano (Institute for Biomedical Research (BIOMED), Catholic University of Argentina (UCA), Buenos Aires, Argentina) for the assistance in mice activity recordings.
Funding
This work was supported by grants from Universidad Nacional de Quilmes (PUNQ 1397/16) and by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PICT 2099–2014) from Argentina. Study design, sample collection, data analysis and interpretation, and writing of the manuscript were performed by the authors with no participation of the funding agencies.
Author information
Authors and Affiliations
Contributions
LLT performed all the experiments and wrote the manuscript. NS assisted in performing the in vivo experiments. JJC was a major contributor in writing the manuscript and directing the study design and data interpretation. PMW served as technical advisor and wrote the manuscript. DAG served as experimental advisor and wrote the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
Protocols with animals were supervised and approved by the Institutional Animal Care and Use Committee of the Universidad Nacional de Quilmes (Protocol resolution 011-15) in accordance with international standards.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Trebucq, L.L., Salvatore, N., Wagner, P.M. et al. Circadian Clock Gene bmal1 Acts as a Tumor Suppressor Gene in a Mice Model of Human Glioblastoma. Mol Neurobiol (2024). https://doi.org/10.1007/s12035-023-03895-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12035-023-03895-7