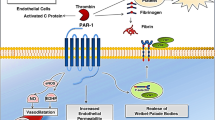
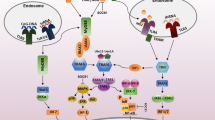
Abstract
In ischemic stroke, there is only one approved drug, tissue plasminogen activator, to be used in clinical conditions for thrombolysis. New neuroprotective therapies for ischemic stroke are desperately needed. Several targets and pathways have been shown to confer neuroprotective effects in ischemic stroke. G-protein-coupled receptors (GPCRs) are one of the most frequently targeted receptors for developing novel therapeutics for central nervous system disorders. GPCRs are a large family of cell surface receptors that response to a wide variety of extracellular stimuli. GPCRs are involved in a wide range of physiological and pathological processes. More than 90% of the identified non-sensory GPCRs are expressed in the brain, where they play important roles in regulating mood, pain, vision, immune responses, cognition, and synaptic transmission. There is also good evidence that GPCRs are implicated in the pathogenesis of stroke. This review narrates the pathophysiological role and possible targeted therapy of GPCRs in ischemic stroke.


Similar content being viewed by others
Data Availability
Not applicable.
Abbreviations
- 2-AG:
-
2-Arachidonoylglycerol
- 2VO:
-
2-vessel occlusion
- ACEA:
-
Arachidonyl-2′-chloroethylamide
- AEA:
-
Anandamide
- AKT:
-
Protein kinase B
- AMPK:
-
AMP-activated protein kinase
- APEC:
-
2-[[2-[4-[2-(2-Aminoethyl)-aminocarbonyl]ethyl]phenyl]ethylamino]-5′-N-ethyl-carboxamidoadenosine
- ATP:
-
Adenosine triphosphate
- BBB:
-
Blood–brain barrier
- BCL-2:
-
B-cell lymphoma 2
- BDNF:
-
Brain-derived neurotrophic factor
- CAD:
-
Coronary artery disease
- cAMP:
-
Cyclic adenosine monophosphate
- CBF:
-
Cerebral blood flow
- CNS:
-
Central nervous system
- COX-2:
-
Cyclooxygenase 2
- CREB:
-
cAMP response element-binding protein
- CRTH2:
-
Chemoattractant receptor T helper type 2
- DAG:
-
Diacylglycerol
- ERK:
-
Extracellular-signal-regulated kinase
- FDA:
-
Food and Drug Administration
- GABA:
-
Gamma-aminobutyric acid
- GCI/R:
-
Global cerebral ischemia/reperfusion
- GDP:
-
Guanine diphosphate
- GluR2:
-
Glutamate receptor subunit 2
- GPCR:
-
G-protein-coupled receptor
- GSK3β:
-
Glycogen synthase kinase 3 beta
- GTP:
-
Guanosine triphosphate
- HIF-1α:
-
Hypoxia-inducible factor 1-alpha
- HI:
-
Hypoxia–ischemia
- HPGDS:
-
Hematopoietic PGD synthase
- HT:
-
Hemorrhagic transformation
- iGluR:
-
Ionotropic glutamate receptor
- IL-1β:
-
Interleukin-1 beta
- IL-6:
-
Interleukin-6
- iNOS:
-
Inducible nitric oxide synthase
- IP3:
-
Inositol triphosphate
- I/R:
-
Ischemia/reperfusion
- LPGDS:
-
Lipocalin-type synthase
- LPS:
-
Lipopolysaccharides
- MAPKs:
-
Mitogen-activated protein kinases
- MCAO:
-
Middle cerebral artery occlusion
- MCP-1:
-
Monocyte chemoattractant protein-1
- mGluR:
-
Metabotropic glutamate receptor
- MMP:
-
Matrix metallopeptidase
- mPTP:
-
Mitochondrial permeability transition pore
- MS:
-
Multiple sclerosis
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NAIP:
-
Neuronal apoptosis inhibitory protein
- NMDA:
-
N-Methyl-D-aspartate
- NF-κB:
-
Nuclear factor kappa B
- NO:
-
Nitric oxide
- OGD:
-
Oxygen glucose deprivation
- OGD/R:
-
Oxygen–glucose deprivation/reperfusion
- PI3K:
-
Phosphoinositide 3-kinases
- PKA:
-
Protein kinase A
- PKCα:
-
Protein kinase C alpha
- PLC:
-
Phospholipase C
- PKCε:
-
Protein kinase C epsilon
- PMCAO:
-
Permanent middle cerebral artery occlusion
- PP1:
-
Protein phosphatase 1
- PSD:
-
Post-stroke depression
- ROS:
-
Reactive oxygen species
- SMCs:
-
Smooth muscle cells
- TBI:
-
Traumatic brain injury
- TGF-β:
-
Transforming growth factor-beta
- TIM-3:
-
T-cell immunoglobulin and mucin domain protein 3
- 5-HT:
-
Serotonin
- tMCAO:
-
Transient middle cerebral artery occlusion
- TNFα:
-
Tumor necrosis factor alpha
- TP:
-
Thromboxane prostanoid
- TPA:
-
Tissue plasminogen activator
- TrkB:
-
Tropomyosin receptor kinase B
- TxB2:
-
Thromboxane B2
- VTA:
-
Ventral tegmental area
References
Gibson CL (2013) Cerebral ischemic stroke: is gender important? J Cereb Blood Flow Metab 33(9):1355–1361
Macrez R, Ali C, Toutirais O, Le Mauff B, Defer G, Dirnagl U, Vivien D (2011) Stroke and the immune system: from pathophysiology to new therapeutic strategies. Lancet Neurol 10(5):471–480
Pérez-Álvarez MJ, del Carmen MM, Anton M, Ordoñez L, Wandosell F (2012) Post-ischemic estradiol treatment reduced glial response and triggers distinct cortical and hippocampal signaling in a rat model of cerebral ischemia. J Neuroinflammation 9(1):157
Vahidinia Z, Tameh AA, Nejati M, Beyer C, Talaei SA, Moghadam SE, Atlasi MA (2019) The protective effect of bone marrow mesenchymal stem cells in a rat model of ischemic stroke via reducing the C-Jun N-terminal kinase expression. Pathology-Research and Practice:152519
Nejati M, Tameh AA, Vahidinia Z, Atlasi MA (2018) Mesenchymal stem cells improve ischemic stroke injury by anti-inflammatory properties in rat model of middle cerebral artery occlusion. Iran Red Crescent Med J 20(1)
Vahidinia Z, Karimian M, Joghataei MT (2020) Neurosteroids and their receptors in ischemic stroke: from molecular mechanisms to therapeutic opportunities. Pharmacological Research:105163
Vahidinia Z, Alipour N, Atlasi MA, Naderian H, Beyer C, Azami Tameh A (2017) Gonadal steroids block the calpain-1-dependent intrinsic pathway of apoptosis in an experimental rat stroke model. Neurol Res 39(1):54–64
Tajalli-Nezhad S, Karimian M, Beyer C, Atlasi MA, Tameh AA (2019) The regulatory role of Toll-like receptors after ischemic stroke: neurosteroids as TLR modulators with the focus on TLR2/4. Cell Mol Life Sci 76(3):523–537
Behdarvandy M, Karimian M, Atlasi MA, Azami Tameh A (2020) Heat shock protein 27 as a neuroprotective biomarker and a suitable target for stem cell therapy and pharmacotherapy in ischemic stroke. Cell Biol Int 44(2):356–367
Schülein R, Westendorf C, Krause G, Rosenthal W (2012) Functional significance of cleavable signal peptides of G protein-coupled receptors. Eur J Cell Biol 91(4):294–299
Dal Prà I, Armato U, Chiarini A (2019) Family C G-protein-coupled receptors in Alzheimer’s disease and therapeutic implications. Front Pharmacol:10
Komatsu H (2015) Novel therapeutic GPCRs for psychiatric disorders. Int J Mol Sci 16(6):14109–14121
Vassilatis DK, Hohmann JG, Zeng H, Li F, Ranchalis JE, Mortrud MT, Brown A, Rodriguez SS et al (2003) The G protein-coupled receptor repertoires of human and mouse. Proc Natl Acad Sci 100(8):4903–4908
McKay EC, Counts SE (2020) Oxytocin receptor signaling in vascular function and stroke. Front Neurosci 14:1003
Guerram M, Zhang L-Y, Jiang Z-Z (2016) G-protein coupled receptors as therapeutic targets for neurodegenerative and cerebrovascular diseases. Neurochem Int 101:1–14
Predescu D-V, Crețoiu SM, Crețoiu D, Pavelescu LA, Suciu N, Radu BM, Voinea S-C (2019) G protein-coupled receptors (GPCRs)-mediated calcium signaling in ovarian cancer: focus on GPCRs activated by neurotransmitters and inflammation-associated molecules. Int J Mol Sci 20(22):5568
Pluimer BR, Colt M, Zhao Z (2020) G protein-coupled receptors in the mammalian blood-brain barrier. Front Cell Neurosci:14
Nickols HH, Conn PJ (2014) Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis 61:55–71
Hauser AS, Attwood MM, Rask-Andersen M, Schiöth HB, Gloriam DE (2017) Trends in GPCR drug discovery: new agents, targets and indications. Nat Rev Drug Discov 16(12):829
Thompson MD, Burnham WM, Cole DE (2005) The G protein-coupled receptors: pharmacogenetics and disease. Crit Rev Clin Lab Sci 42(4):311–389
Moghadam SE, Tameh AA, Vahidinia Z, Atlasi MA, Bafrani HH, Naderian H (2018) Neuroprotective effects of oxytocin hormone after an experimental stroke model and the possible role of calpain-1. J Stroke Cerebrovasc Dis 27(3):724–732
Karelina K, Stuller KA, Jarrett B, Zhang N, Wells J, Norman GJ, DeVries AC (2011) Oxytocin mediates social neuroprotection after cerebral ischemia. Stroke 42(12):3606–3611
Wess J, Eglen RM, Gautam D (2007) Muscarinic acetylcholine receptors: mutant mice provide new insights for drug development. Nat Rev Drug Discov 6(9):721–733
Filip M, Bader M (2009) Overview on 5-HT receptors and their role in physiology and pathology of the central nervous system. Pharmacol Rep 61(5):761–777
Vitalis T, Parnavelas JG (2003) The role of serotonin in early cortical development. Dev Neurosci 25(2-4):245–256
Schmitz D, Empson R, Heinemann U (1995) Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Res 701(1-2):249–254
Globus M, Wester P, Busto R, Dietrich WD (1992) Ischemia-induced extracellular release of serotonin plays a role in CA1 neuronal cell death in rats. Stroke 23(11):1595–1601
Yi C-A, Wang J, Wang Y, Wu X-Y (2019) Neuroprotection by 2, 3, 5, 4′-tetrahydroxystilbene-2-O-β-D-glucoside extracts from Polygonum multiflorum against cerebral ischemia/reperfusion injury through the 5-hydroxytryptamine/5-hydroxytryptamine receptor pathway. Neuropsychiatr Dis Treat 15:1429
Kanthan R, Shuaib A, Griebel R, El-Alazounni H, Miyashita H, Kalra J (1996) Evaluation of monoaminergic neurotransmitters in the acute focal ischemic human brain model by intracerebral in vivo microdialysis. Neurochem Res 21(5):563–566
Allen GS, Gold LH, Chou SN, French LA (1974) Cerebral arterial spasm. Part 3. In vivo intracisternal production of spasm by serotonin and blood and its reversal by phenoxybenzamine. J Neurosurg 40(4):451–458
Cao L, Xu C-B, Zhang Y, Cao Y, Edvinsson L (2013) Secondhand cigarette smoke exposure causes upregulation of cerebrovascular 5-HT 1 B receptors via the R af/ERK/MAPK pathway in rats. Acta Physiol 207(1):183–193
Doggrell SA (2004) Sarpogrelate: cardiovascular and renal clinical potential. Expert Opin Investig Drugs 13(7):865–874
Ban Y, Watanabe T, Miyazaki A, Nakano Y, Tobe T, Idei T, Iguchi T, Ban Y et al (2007) Impact of increased plasma serotonin levels and carotid atherosclerosis on vascular dementia. Atherosclerosis 195(1):153–159
Hara K, Hirowatari Y, Yoshika M, Komiyama Y, Tsuka Y, Takahashi H (2004) The ratio of plasma to whole-blood serotonin may be a novel marker of atherosclerotic cardiovascular disease. J Lab Clin Med 144(1):31–37
Lee CH, Park JH, Yoo K-Y, Choi JH, Hwang IK, Ryu PD, Kim D-H, Kwon Y-G et al (2011) Pre-and post-treatments with escitalopram protect against experimental ischemic neuronal damage via regulation of BDNF expression and oxidative stress. Exp Neurol 229(2):450–459
Kim DH, Li H, Yoo K-Y, Lee B-H, Hwang IK, Won MH (2007) Effects of fluoxetine on ischemic cells and expressions in BDNF and some antioxidants in the gerbil hippocampal CA1 region induced by transient ischemia. Exp Neurol 204(2):748–758
Nichols DE, Nichols CD (2008) Serotonin receptors. Chem Rev 108(5):1614–1641
Leiser SC, Li Y, Pehrson AL, Dale E, Smagin G, Sanchez C (2015) Serotonergic regulation of prefrontal cortical circuitries involved in cognitive processing: a review of individual 5-HT receptor mechanisms and concerted effects of 5-HT receptors exemplified by the multimodal antidepressant vortioxetine. ACS Chem Neurosci 6(7):970–986
Carr DB, Cooper DC, Ulrich SL, Spruston N, Surmeier DJ (2002) Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. J Neurosci 22(16):6846–6855
Torup L, Møller A, Sager TN, Diemer NH (2000) Neuroprotective effect of 8-OH-DPAT in global cerebral ischemia assessed by stereological cell counting. Eur J Pharmacol 395(2):137–141
Schiapparelli L, Del Río J, Frechilla D (2005) Serotonin 5-HT1A receptor blockade enhances Ca2+/calmodulin-dependent protein kinase II function and membrane expression of AMPA receptor subunits in the rat hippocampus: implications for memory formation. J Neurochem 94(4):884–895
Salazar-Colocho P, Del Río J, Frechilla D (2008) Neuroprotective effects of serotonin 5-HT1A receptor activation against ischemic cell damage in gerbil hippocampus: Involvement of NMDA receptor NR1 subunit and BDNF. Brain Res 1199:159–166
Aguiar RP, Soares LM, Meyer E, da Silveira FC, Milani H, Newman-Tancredi A, Varney M, Prickaerts J et al (2020) Activation of 5-HT1A postsynaptic receptors by NLX-101 results in functional recovery and an increase in neuroplasticity in mice with brain ischemia. Prog Neuro-Psychopharmacol Biol Psychiatry 99:109832
Fogaça MV, Campos AC, Coelho LD, Duman RS, Guimarães FS (2018) The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: role of neurogenesis and dendritic remodeling. Neuropharmacology 135:22–33
Marco I, Valhondo M, Martı́n-Fontecha M, Vázquez-Villa H, Del Rı́o Jn, Planas A, Sagredo O, Ramos JA, Torrecillas InR, Pardo L (2011) New serotonin 5-HT1A receptor agonists with neuroprotective effect against ischemic cell damage. J Med Chem 54 (23):7986-7999
Kukley M, Schaper C, Becker A, Rose K, Krieglstein J (2001) Effect of 5-hydroxytryptamine 1A receptor agonist BAY X 3702 on BCL-2 and BAX proteins level in the ipsilateral cerebral cortex of rats after transient focal ischaemia. Neuroscience 107(3):405–413
Adayev T, Ray I, Sondhi R, Sobocki T, Banerjee P (2003) The G protein-coupled 5-HT1A receptor causes suppression of caspase-3 through MAPK and protein kinase Cα. Biochim Biophys Acta (BBA)-Mol Cell Res 1640(1):85–96
Johansen FF, Hasseldam H, Smith MN, Rasmussen RS (2014) Drug-induced hypothermia by 5HT1A agonists provide neuroprotection in experimental stroke: new perspectives for acute patient treatment. J Stroke Cerebrovasc Dis 23(10):2879–2887
Iannuzzi N, Liebeskind D, Jacoby M, Arima K, Shimizu K, Zimmerman T (2006) Piclozotan (SUN N4057), a novel 5HT1A receptor agonist, is well tolerated in patients with acute stroke: P39. Stroke 37(2)
Teal P, Davis S, Hacke W, Kaste M, Lyden PD, Fierus M, AG BH (2009) A randomized, double-blind, placebo-controlled trial to evaluate the efficacy, safety, tolerability, and pharmacokinetic/pharmacodynamic effects of a targeted exposure of intravenous repinotan in patients with acute ischemic stroke: modified randomized exposure controlled trial (mRECT). Stroke 40 (11):3518-3525
Allen GS, Henderson LM, Chou SN, French LA (1974) Cerebral arterial spasm. Part 2. In vitro contractile activity of serotonin in human serum and CSF on the canine basilar artery, and its blockage by methylsergide and phenoxybenzamine. J Neurosurg 40(4):442–450
Villalón CM, Centurión D (2007) Cardiovascular responses produced by 5-hydroxytriptamine: a pharmacological update on the receptors/mechanisms involved and therapeutic implications. Naunyn Schmiedeberg's Arch Pharmacol 376(1-2):45–63
Hoel NL, Hansen-Schwartz J, Edvinsson L (2001) Selective up-regulation of 5-HT1B/1D receptors during organ culture of cerebral arteries. Neuroreport 12(8):1605–1608
Nilsson T, Longmore J, Shaw D, Olesen IJ, Edvinsson L (1999) Contractile 5-HT1B receptors in human cerebral arteries: pharmacological characterization and localization with immunocytochemistry. Br J Pharmacol 128(6):1133–1140
Maddahi A, Edvinsson L (2008) Enhanced expressions of microvascular smooth muscle receptors after focal cerebral ischemia occur via the MAPK MEK/ERK pathway. BMC Neurosci 9(1):85
Vikman P, Edvinsson L (2006) Gene expression profiling in the human middle cerebral artery after cerebral ischemia. Eur J Neurol 13(12):1324–1332
Rasmussen MN, Hornbak M, Larsen SS, Sheykhzade M, Edvinsson L (2013) Permanent distal occlusion of middle cerebral artery in rat causes local increased ETB, 5-HT1B and AT1 receptor-mediated contractility downstream of occlusion. J Vasc Res 50(5):396–409
Uchiyama S, Ozaki Y, Satoh K, Kondo K, Nishimaru K (2007) Effect of sarpogrelate, a 5-HT2A antagonist, on platelet aggregation in patients with ischemic stroke: clinical-pharmacological dose-response study. Cerebrovasc Dis 24(2-3):264–270
Sakurai-Yamashita Y, Yamashita K, Niwa M, Taniyama K (2003) Involvement of 5-hydroxytryptamine4 receptor in the exacerbation of neuronal loss by psychological stress in the hippocampus of SHRSP with a transient ischemia. Brain Res 973(1):92–98
Schultz W (2007) Multiple dopamine functions at different time courses. Annu Rev Neurosci 30:259–288
Bozzi Y, Borrelli E (2006) Dopamine in neurotoxicity and neuroprotection: what do D2 receptors have to do with it? Trends Neurosci 29(3):167–174
Floel A, Hummel F, Breitenstein C, Knecht S, Cohen L (2005) Dopaminergic effects on encoding of a motor memory in chronic stroke. Neurology 65(3):472–474
Kuric E, Ruscher K (2014) Reduction of rat brain CD 8+ T-cells by levodopa/benserazide treatment after experimental stroke. Eur J Neurosci 40(2):2463–2470
Gao B, Cam E, Jaeger H, Zunzunegui C, Sarnthein J, Bassetti CL (2010) Sleep disruption aggravates focal cerebral ischemia in the rat. Sleep 33(7):879–887
Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti M-S, Luft AR (2009) Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One 4(9):e7082
Blandini F, Armentero M-T (2014) Dopamine receptor agonists for Parkinson’s disease. Expert Opin Investig Drugs 23(3):387–410
Contreras F, Fouillioux C, Bolívar A, Simonovis N, Hernández-Hernández R, Armas-Hernandez M, Velasco M (2002) Dopamine, hypertension and obesity. J Hum Hypertens 16(1):S13–S17
Svenningsson P, Nishi A, Fisone G, Girault J-A, Nairn AC, Greengard P (2004) DARPP-32: an integrator of neurotransmission. Annu Rev Pharmacol Toxicol 44:269–296
Yamamoto Y, Tanaka T, Shibata S, Watanabe S (1994) Involvement of D1 dopamine receptor mechanism in ischemia-induced impairment of CA1 presynaptic fiber spikes in rat hippocampal slices. Brain Res 665(1):151–154
Okada Y, Sakai H, Kohiki E, Suga E, Yanagisawa Y, Tanaka K, Hadano S, Osuga H et al (2005) A dopamine D4 receptor antagonist attenuates ischemia-induced neuronal cell damage via upregulation of neuronal apoptosis inhibitory protein. J Cereb Blood Flow Metab 25(7):794–806
Zou S, Li L, Pei L, Vukusic B, Van Tol HH, Lee FJ, Wan Q, Liu F (2005) Protein-protein coupling/uncoupling enables dopamine D2 receptor regulation of AMPA receptor-mediated excitotoxicity. J Neurosci 25(17):4385–4395
Hisahara S (2011) Shimohama S (2011) Dopamine receptors and Parkinson’s disease. Int J Med Chem
Baik J-H, Picetti R, Saiardi A, Thiriet G, Dierich A, Depaulis A, Le Meur M, Borrelli E (1995) Parkinsonian-like locomotor impairment in mice lacking dopamine D2 receptors. Nature 377(6548):424–428
Huck JH, Freyer D, Böttcher C, Mladinov M, Muselmann-Genschow C, Thielke M, Gladow N, Bloomquist D et al (2015) De novo expression of dopamine D2 receptors on microglia after stroke. J Cereb Blood Flow Metab 35(11):1804–1811
Kihara T, Shimohama S, Sawada H, Honda K, Nakamizo T, Kanki R, Yamashita H, Akaike A (2002) Protective effect of dopamine D2 agonists in cortical neurons via the phosphatidylinositol 3 kinase cascade. J Neurosci Res 70(3):274–282
O’Neill MJ, Hicks CA, Ward MA, Cardwell GP, Reymann J-M, Allain H, Bentué-Ferrer D (1998) Dopamine D2 receptor agonists protect against ischaemia-induced hippocampal neurodegeneration in global cerebral ischaemia. Eur J Pharmacol 352(1):37–46
Andrabi SS, Tabassum H, Parveen S, Parvez S (2020) Ropinirole induces neuroprotection following reperfusion-promoted mitochondrial dysfunction after focal cerebral ischemia in Wistar rats. Neurotoxicology 77:94–104
Yang Z-J, Torbey M, Li X, Bernardy J, Golden WC, Martin LJ, Koehler RC (2007) Dopamine receptor modulation of hypoxic—ischemic neuronal injury in striatum of newborn piglets. J Cereb Blood Flow Metab 27(7):1339–1351
Hall ED, Andrus PK, Oostveen JA, Althaus JS, Von Voigtlander PF (1996) Neuroprotective effects of the dopamine D2/D3 agonist pramipexole against postischemic or methamphetamine-induced degeneration of nigrostriatal neurons. Brain Res 742(1-2):80–88
Wang W, Liu L, Chen C, Jiang P, Zhang T (2018) Protective effects of dopamine D2/D3 receptor agonist piribedil on learning and memory of rats exposed to global cerebral ischemia–reperfusion. Neurosci Lett 684:181–186
Rosin C, Colombo S, Calver AA, Bates TE, Skaper SD (2005) Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia 52(4):336–343
Sethy VH, Wu H, Oostveen JA, Hall ED (1997) Neuroprotective effects of the dopamine agonists pramipexole and bromocriptine in 3-acetylpyridine-treated rats. Brain Res 754(1-2):181–186
Ling ZD, Robie HC, Tong CW, Carvey PM (1999) Both the antioxidant and D3 agonist actions of pramipexole mediate its neuroprotective actions in mesencephalic cultures. J Pharmacol Exp Ther 289(1):202–210
Takashima H, Tsujihata M, Kishikawa M, Freed WJ (1999) Bromocriptine protects dopaminergic neurons from levodopa-induced toxicity by stimulating D2Receptors. Exp Neurol 159(1):98–104
Borovac JA (2016) Focus: The aging brain: side effects of a dopamine agonist therapy for Parkinson’s disease: a mini-review of clinical pharmacology. Yale J Biol Med 89(1):37
Landucci E, Scartabelli T, Gerace E, Moroni F, Pellegrini-Giampietro DE (2011) CB1 receptors and post-ischemic brain damage: studies on the toxic and neuroprotective effects of cannabinoids in rat organotypic hippocampal slices. Neuropharmacology 60(4):674–682
Zhang M, Adler MW, Abood ME, Ganea D, Jallo J, Tuma RF (2009) CB2 receptor activation attenuates microcirculatory dysfunction during cerebral ischemic/reperfusion injury. Microvasc Res 78(1):86–94
Bénard G, Massa F, Puente N, Lourenço J, Bellocchio L, Soria-Gómez E, Matias I, Delamarre A et al (2012) Mitochondrial CB 1 receptors regulate neuronal energy metabolism. Nat Neurosci 15(4):558
Stornaiuolo M, Bruno A, Botta L, La Regina G, Cosconati S, Silvestri R, Marinelli L, Novellino E (2015) Endogenous vs exogenous allosteric modulators in GPCRs: a dispute for shuttling CB 1 among different membrane microenvironments. Sci Rep 5:15453
Pazos MR, Núñez E, Benito C, Tolón RM, Romero J (2005) Functional neuroanatomy of the endocannabinoid system. Pharmacol Biochem Behav 81(2):239–247
Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN (2005) Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. J Neurochem 95(2):437–445
Lynch DL, Hurst DP, Reggio PH (2020) The nucleotide-free state of the cannabinoid CB2/Gi complex. Cell 180(4):603–604
Suzuki N, Suzuki M, Murakami K, Hamajo K, Tsukamoto T, Shimojo M (2012) Cerebroprotective effects of TAK-937, a cannabinoid receptor agonist, on ischemic brain damage in middle cerebral artery occluded rats and non-human primates. Brain Res 1430:93–100
Ashton JC, Rahman RM, Nair SM, Sutherland BA, Glass M, Appleton I (2007) Cerebral hypoxia-ischemia and middle cerebral artery occlusion induce expression of the cannabinoid CB2 receptor in the brain. Neurosci Lett 412(2):114–117
Jin KL, Mao XO, Goldsmith PC, Greenberg DA (2000) CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol 48(2):257–261
Sun S, Chen X, Gao Y, Liu Z, Zhai Q, Xiong L, Cai M, Wang Q (2016) Mn-SOD upregulation by electroacupuncture attenuates ischemic oxidative damage via CB1R-mediated STAT3 phosphorylation. Mol Neurobiol 53(1):331–343
Knowles MD, de la Tremblaye PB, Azogu I, Plamondon H (2016) Endocannabinoid CB1 receptor activation upon global ischemia adversely impact recovery of reward and stress signaling molecules, neuronal survival and behavioral impulsivity. Prog Neuro-Psychopharmacol Biol Psychiatry 66:8–21
Cai M, Yang Q, Li G, Sun S, Chen Y, Tian L, Dong H (2017) Activation of cannabinoid receptor 1 is involved in protection against mitochondrial dysfunction and cerebral ischaemic tolerance induced by isoflurane preconditioning. BJA Br J Anaesth 119(6):1213–1223
Ma L, Jia J, Niu W, Jiang T, Zhai Q, Yang L, Bai F, Wang Q et al (2015) Mitochondrial CB1 receptor is involved in ACEA-induced protective effects on neurons and mitochondrial functions. Sci Rep 5(1):1–13
Caltana L, Saez TM, Aronne MP, Brusco A (2015) Cannabinoid receptor type 1 agonist ACEA improves motor recovery and protects neurons in ischemic stroke in mice. J Neurochem 135(3):616–629
Ma L, Niu W, Yang S, Tian J, Luan H, Cao M, Xi W, Tu W et al (2018) Inhibition of mitochondrial permeability transition pore opening contributes to cannabinoid type 1 receptor agonist ACEA-induced neuroprotection. Neuropharmacology 135:211–222
Bai F, Guo F, Jiang T, Wei H, Zhou H, Yin H, Zhong H, Xiong L et al (2017) Arachidonyl-2-chloroethylamide alleviates cerebral ischemia injury through glycogen synthase kinase-3β-mediated mitochondrial biogenesis and functional improvement. Mol Neurobiol 54(2):1240–1253
Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L (2009) Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke 40(6):2157–2164
Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao Z, Marsicano G, Wang Q et al (2013) Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res 1529:154–164
Wei H, Yao X, Yang L, Wang S, Guo F, Zhou H, Marsicano G, Wang Q et al (2014) Glycogen synthase kinase-3β is involved in electroacupuncture pretreatment via the cannabinoid CB1 receptor in ischemic stroke. Mol Neurobiol 49(1):326–336
Liu Z, Chen X, Gao Y, Sun S, Yang L, Yang Q, Bai F, Xiong L et al (2015) Involvement of GluR2 up-regulation in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptor in mice. Sci Rep 5:9490
Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA (2002) Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci 22(22):9771–9775
Chi OZ, Barsoum S, Grayson J, Hunter C, Liu X, Weiss HR (2012) Effects of cannabinoid receptor agonist WIN 55,212-2 on blood-brain barrier disruption in focal cerebral ischemia in rats. Pharmacology 89(5-6):333–338
Murikinati S, Jüttler E, Keinert T, Ridder DA, Muhammad S, Waibler Z, Ledent C, Zimmer A et al (2010) Activation of cannabinoid 2 receptors protects against cerebral ischemia by inhibiting neutrophil recruitment. FASEB J 24(3):788–798
Ronca RD, Myers AM, Ganea D, Tuma RF, Walker EA, Ward SJ (2015) A selective cannabinoid CB2 agonist attenuates damage and improves memory retention following stroke in mice. Life Sci 138:72–77
Jayant S, Sharma B (2016) Selective modulator of cannabinoid receptor type 2 reduces memory impairment and infarct size during cerebral hypoperfusion and vascular dementia. Curr Neurovasc Res 13(4):289–302
Choi I-Y, Ju C, Jalin AMA, Lee DI, Prather PL, Kim W-K (2013) activation of cannabinoid CB2 receptor–mediated AMPK/CREB pathway reduces cerebral ischemic injury. Am J Pathol 182(3):928–939
Guo K, Mou X, Huang J, Xiong N, Li H (2014) Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. J Mol Neurosci 54(1):41–48
Kossatz E, Maldonado R, Robledo P (2016) CB2 cannabinoid receptors modulate HIF-1α and TIM-3 expression in a hypoxia-ischemia mouse model. Eur Neuropsychopharmacol 26(12):1972–1988
Garcia-Ovejero D, Arevalo-Martin A, Petrosino S, Docagne F, Hagen C, Bisogno T, Watanabe M, Guaza C et al (2009) The endocannabinoid system is modulated in response to spinal cord injury in rats. Neurobiol Dis 33(1):57–71
Contartese A, Valoti M, Corelli F, Pasquini S, Mugnaini C, Pessina F, Aldinucci C, Sgaragli G et al (2012) A novel CB2 agonist, COR167, potently protects rat brain cortical slices against OGD and reperfusion injury. Pharmacol Res 66(6):555–563
Grundy RI (2002) The therapeutic potential of the cannabinoids in neuroprotection. Expert Opin Investig Drugs 11(10):1365–1374
Zhuang S-Y, Bridges D, Grigorenko E, McCloud S, Boon A, Hampson RE, Deadwyler SA (2005) Cannabinoids produce neuroprotection by reducing intracellular calcium release from ryanodine-sensitive stores. Neuropharmacology 48(8):1086–1096
Hu B, Wang Q, Chen Y, Du J, Zhu X, Lu Y, Xiong L, Chen S (2010) Neuroprotective effect of WIN 55,212-2 pretreatment against focal cerebral ischemia through activation of extracellular signal-regulated kinases in rats. Eur J Pharmacol 645(1-3):102–107
Sun J, Y-q F, Ren H, Chen T, Guo J-j, Yan J, Song S, L-y Z et al (2013) WIN55, 212-2 protects oligodendrocyte precursor cells in stroke penumbra following permanent focal cerebral ischemia in rats. Acta Pharmacol Sin 34(1):119–128
Wang D-P, Yin H, Kang K, Lin Q, Su S-H, Hai J (2018) The potential protective effects of cannabinoid receptor agonist WIN55, 212-2 on cognitive dysfunction is associated with the suppression of autophagy and inflammation in an experimental model of vascular dementia. Psychiatry Res 267:281–288
Muthian S, Rademacher D, Roelke C, Gross G, Hillard C (2004) Anandamide content is increased and CB1 cannabinoid receptor blockade is protective during transient, focal cerebral ischemia. Neuroscience 129(3):743–750
Hansen HH, Azcoitia I, Pons S, Romero J, García-Segura LM, Ramos JA, Hansen HS, Fernández-Ruiz J (2002) Blockade of cannabinoid CB1 receptor function protects against in vivo disseminating brain damage following NMDA-induced excitotoxicity. J Neurochem 82(1):154–158
Jalin AMA, Rajasekaran M, Prather PL, Kwon JS, Gajulapati V, Choi Y, Kim C, Pahk K et al (2015) Non-selective cannabinoid receptor antagonists, hinokiresinols reduce infiltration of microglia/macrophages into ischemic brain lesions in rat via modulating 2-arachidonolyglycerol-induced migration and mitochondrial activity. PLoS One 10(10)
Rivers-Auty J, Smith P, Ashton J (2014) The cannabinoid CB2 receptor agonist GW405833 does not ameliorate brain damage induced by hypoxia-ischemia in rats. Neurosci Lett 569:104–109
Carvajal FJ, Mattison HA, Cerpa W (2016) Role of NMDA receptor-mediated glutamatergic signaling in chronic and acute neuropathologies. Neural Plast:2016
Vahidinia Z, Mahdavi E, Talaei SA, Naderian H, Tamtaji A, Kashani HH, Beyer C, Tameh AA (2021) The effect of female sex hormones on Hsp27 phosphorylation and histological changes in prefrontal cortex after tMCAO. Pathology-Research and Practice:153415
Yoshioka H, Sugita M, Kinouchi H (2009) Neuroprotective effects of group II metabotropic glutamate receptor agonist DCG-IV on hippocampal neurons in transient forebrain ischemia. Neurosci Lett 461(3):266–270
Nematipour S, Vahidinia Z, Nejati M, Naderian H, Beyer C, Tameh AA (2020) Estrogen and progesterone attenuate glutamate neurotoxicity via regulation of EAAT3 and GLT-1 in a rat model of ischemic stroke. Iran J Basic Med Sci 23(10):1346
Szatkowski M, Attwell D (1994) Triggering and execution of neuronal death in brain ischaemia: two phases of glutamate release by different mechanisms. Trends Neurosci 17(9):359–365
Tameh AA, Karimian M, Zare-Dehghanani Z, Aftabi Y, Beyer C (2018) Role of steroid therapy after ischemic stroke by n-methyl-d-aspartate receptor gene regulation. J Stroke Cerebrovasc Dis 27(11):3066–3075
Niu H-X, Wang J-Z, Wang D-L, Miao J-J, Li H, Liu Z-G, Yuan X, Liu W et al (2018) The orally active noncompetitive AMPAR antagonist perampanel attenuates focal cerebral ischemia injury in rats. Cell Mol Neurobiol 38(2):459–466
Fan H, Li X, Wang W, Lai Q, Tang X, Gao D, Yin X, Xu T (2015) Effects of NMDA-receptor antagonist on the expressions of Bcl-2 and Bax in the subventricular zone of neonatal rats with hypoxia–ischemia brain damage. Cell Biochem Biophys 73(2):323–330
Neuhaus AA, Rabie T, Sutherland BA, Papadakis M, Hadley G, Cai R, Buchan AM (2014) Importance of preclinical research in the development of neuroprotective strategies for ischemic stroke. JAMA Neurol 71(5):634–639
Grupke S, Hall J, Dobbs M, Bix GJ, Fraser JF (2015) Understanding history, and not repeating it. Neuroprotection for acute ischemic stroke: from review to preview. Clin Neurol Neurosurg 129:1–9
Bruno V, Battaglia G, Copani A, D’Onofrio M, Di Iorio P, De Blasi A, Melchiorri D, Flor PJ et al (2001) Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J Cereb Blood Flow Metab 21(9):1013–1033
Fröhlich F (2016) Network neuroscience. Academic Press
Spooren W, Ballard T, Gasparini F, Amalric M, Mutel V, Schreiber R (2003) Insight into the function of group I and group II metabotropic glutamate (mGlu) receptors: behavioural characterization and implications for the treatment of CNS disorders. Behav Pharmacol 14(4):257–277
Nakajima Y, Iwakabe H, Akazawa C, Nawa H, Shigemoto R, Mizuno N, Nakanishi S (1993) Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J Biol Chem 268(16):11868–11873
Cavallo D, Landucci E, Gerace E, Lana D, Ugolini F, Henley JM, Giovannini MG, Pellegrini-Giampietro DE (2020) Neuroprotective effects of mGluR5 activation through the PI3K/Akt pathway and the molecular switch of AMPA receptors. Neuropharmacology 162:107810
Kohara A, Takahashi M, S-i Y, Tamura S, Shitaka Y, Hayashibe S, Kawabata S, Okada M (2008) Neuroprotective effects of the selective type 1 metabotropic glutamate receptor antagonist YM-202074 in rat stroke models. Brain Res 1191:168–179
Makarewicz D, Duszczyk M, Gadamski R, Danysz W, Łazarewicz JW (2006) Neuroprotective potential of group I metabotropic glutamate receptor antagonists in two ischemic models. Neurochem Int 48(6-7):485–490
Szydlowska K, Kaminska B, Baude A, Parsons CG, Danysz W (2007) Neuroprotective activity of selective mGlu1 and mGlu5 antagonists in vitro and in vivo. Eur J Pharmacol 554(1):18–29
Faden AI, O’Leary DM, Fan L, Bao W, Mullins PG, Movsesyan VA (2001) Selective blockade of the mGluR1 receptor reduces traumatic neuronal injury in vitro and improves outcome after brain trauma. Exp Neurol 167(2):435–444
Pellegrini-Giampietro DE, Peruginelli F, Meli E, Cozzi A, Albani-Torregrossa S, Pellicciari R, Moroni F (1999) Protection with metabotropic glutamate 1 receptor antagonists in models of ischemic neuronal death: time-course and mechanisms. Neuropharmacology 38(10):1607–1619
Murotomi K, Takagi N, Takayanagi G, Ono M, Takeo S, Tanonaka K (2008) mGluR1 antagonist decreases tyrosine phosphorylation of NMDA receptor and attenuates infarct size after transient focal cerebral ischemia. J Neurochem 105(5):1625–1634
Murotomi K, Takagi N, Mizutani R, Ta H, Ono M, Takeo S, Tanonaka K (2010) mGluR1 antagonist decreased NADPH oxidase activity and superoxide production after transient focal cerebral ischemia. J Neurochem 114(6):1711–1719
Battaglia G, Bruno V, Pisani A, Centonze D, Catania MV, Calabresi P, Nicoletti F (2001) Selective blockade of type-1 metabotropic glutamate receptors induces neuroprotection by enhancing gabaergic transmission. Mol Cell Neurosci 17(6):1071–1083
Ye X, Yu L, Zuo D, Zhang L, Zu J, Hu J, Tang J, Bao L et al (2017) Activated mGluR5 protects BV2 cells against OGD/R induced cytotoxicity by modulating BDNF-TrkB pathway. Neurosci Lett 654:70–79
Byrnes KR, Stoica B, Loane DJ, Riccio A, Davis MI, Faden AI (2009) Metabotropic glutamate receptor 5 activation inhibits microglial associated inflammation and neurotoxicity. Glia 57(5):550–560
Takagi N, Besshoh S, Morita H, Terao M, Takeo S, Tanonaka K (2010) Metabotropic glutamate mGlu5 receptor-mediated serine phosphorylation of NMDA receptor subunit NR1 in hippocampal CA1 region after transient global ischemia in rats. Eur J Pharmacol 644(1-3):96–100
Takagi N, Besshoh S, Marunouchi T, Takeo S, Tanonaka K (2012) Effects of metabotropic glutamate mGlu5 receptor antagonist on tyrosine phosphorylation of NMDA receptor subunits and cell death in the hippocampus after brain ischemia in rats. Neurosci Lett 530(1):91–96
Bao W, Williams A, Faden A, Tortella F (2001) Selective mGluR5 receptor antagonist or agonist provides neuroprotection in a rat model of focal cerebral ischemia. Brain Res 922(2):173–179
Riek-Burchardt M, Henrich-Noack P, Reymann K (2007) No improvement of functional and histological outcome after application of the metabotropic glutamate receptor 5 agonist CHPG in a model of endothelin-1-induced focal ischemia in rats. Neurosci Res 57(4):499–503
Li H, Zhang N, Sun G, Ding S (2013) Inhibition of the group I mGluRs reduces acute brain damage and improves long-term histological outcomes after photothrombosis-induced ischaemia. ASN Neuro 5(3):AN20130002
Imre G (2007) The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev 13(4):444–464
Pinheiro PS, Mulle C (2008) Presynaptic glutamate receptors: physiological functions and mechanisms of action. Nat Rev Neurosci 9(6):423–436
Bratek E, Ziembowicz A, Bronisz A, Salinska E (2018) The activation of group II metabotropic glutamate receptors protects neonatal rat brains from oxidative stress injury after hypoxia-ischemia. PLoS One 13(7)
Bratek E, Ziembowicz A, Salinska E (2018) Pretreatment with group II metabotropic glutamate receptor agonist LY379268 protects neonatal rat brains from oxidative stress in an experimental model of birth asphyxia. Brain Sci 8(3):48
Bond A, Ragumoorthy N, Monn JA, Hicks CA, Ward MA, Lodge D, O’Neill MJ (1999) LY379268, a potent and selective group II metabotropic glutamate receptor agonist, is neuroprotective in gerbil global, but not focal, cerebral ischaemia. Neurosci Lett 273(3):191–194
Bond A, O’Neill MJ, Hicks CA, Monn JA, Lodge D (1998) Neuroprotective effects of a systemically active group II metabotropic glutamate receptor agonist LY354740 in a gerbil model of global ischaemia. Neuroreport 9(6):1191–1193
Corti C, Battaglia G, Molinaro G, Riozzi B, Pittaluga A, Corsi M, Mugnaini M, Nicoletti F et al (2007) The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J Neurosci 27(31):8297–8308
Mastroiacovo F, Moyanova S, Cannella M, Gaglione A, Verhaeghe R, Bozza G, Madonna M, Motolese M et al (2017) Genetic deletion of mGlu2 metabotropic glutamate receptors improves the short-term outcome of cerebral transient focal ischemia. Mol Brain 10(1):39
Motolese M, Mastroiacovo F, Cannella M, Bucci D, Gaglione A, Riozzi B, Lütjens R, Poli SM et al (2015) Targeting type-2 metabotropic glutamate receptors to protect vulnerable hippocampal neurons against ischemic damage. Mol Brain 8(1):66
Motolese M, Mastroiacovo F, Cannella M, Bucci D, Gaglione A, Riozzi B, Lütjens R, Poli SM et al (2015) Targeting type-2 metabotropic glutamate receptors to protect vulnerable hippocampal neurons against ischemic damage. Mol Brain 8(1):1–13
Cai Z, Lin S, Rhodes PG (2002) Neuroprotective effects of N-acetylaspartylglutamate in a neonatal rat model of hypoxia-ischemia. Eur J Pharmacol 437(3):139–145
Van Hemelrijck A, Hachimi-Idrissi S, Sarre S, Ebinger G, Michotte Y (2005) Neuroprotective effect of N-acetyl-aspartyl-glutamate in combination with mild hypothermia in the endothelin-1 rat model of focal cerebral ischaemia. J Neurochem 95(5):1287–1297
Bruno V, Battaglia G, Casabona G, Copani A, Caciagli F, Nicoletti F (1998) Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-β. J Neurosci 18(23):9594–9600
Domin H, Przykaza Ł, Jantas D, Kozniewska E, Boguszewski PM, Śmiałowska M (2016) Neuroprotective potential of the group III mGlu receptor agonist ACPT-I in animal models of ischemic stroke: in vitro and in vivo studies. Neuropharmacology 102:276–294
Domin H, Przykaza Ł, Kozniewska E, Boguszewski PM, Śmiałowska M (2018) Neuroprotective effect of the group III mGlu receptor agonist ACPT-I after ischemic stroke in rats with essential hypertension. Prog Neuro-Psychopharmacol Biol Psychiatry 84:93–101
Conn PJ, Pin J-P (1997) Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol 37(1):205–237
Bradley SR, Levey AI, Hersch SM, Conn PJ (1996) Immunocytochemical localization of group III metabotropic glutamate receptors in the hippocampus with subtype-specific antibodies. J Neurosci 16(6):2044–2056
Schoepp DD (2001) Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J Pharmacol Exp Ther 299(1):12–20
Bruno V, Battaglia G, Ksiazek I, Van der Putten H, Catania M, Giuffrida R, Lukic S, Leonhardt T et al (2000) Selective activation of mGlu4 metabotropic glutamate receptors is protective against excitotoxic neuronal death. J Neurosci 20(17):6413–6420
Gasparini F, Bruno V, Battaglia G, Lukic S, Leonhardt T, Inderbitzin W, Laurie D, Sommer B et al (1999) (R, S)-4-phosphonophenylglycine, a potent and selective group III metabotropic glutamate receptor agonist, is anticonvulsive and neuroprotective in vivo. J Pharmacol Exp Ther 289(3):1678–1687
Moyanova SG, Mastroiacovo F, Kortenska LV, Mitreva RG, Fardone E, Santolini I, Sobrado M, Battaglia G et al (2011) Protective role for type 4 metabotropic glutamate receptors against ischemic brain damage. J Cereb Blood Flow Metab 31(4):1107–1118
Rosdahl D, Seitzberg DA, Christensen T, Balchen T, Diemer NH (1994) Changes in mRNA for metabotropic glutamate receptors after transient cerebral ischaemia. Neuroreport 5(5):593–596
Iversen L, Mulvihill E, Haldeman B, Diemer NH, Kaiser F, Sheardown M, Kristensen P (1994) Changes in metabotropic glutamate receptor mRNA levels following global ischemia: increase of a putative presynaptic subtype (mGluR4) in highly vulnerable rat brain areas. J Neurochem 63(2):625–633
Hogan YH, Hawkins R, Alkadhi KA (1998) Adenosine A1 receptor activation inhibits LTP in sympathetic ganglia. Brain Res 807(1-2):19–28
Chen J-F, Xu K, Petzer JP, Staal R, Xu Y-H, Beilstein M, Sonsalla PK, Castagnoli K et al (2001) Neuroprotection by caffeine and A2A adenosine receptor inactivation in a model of Parkinson’s disease. J Neurosci 21(10):RC143–RC143
Fredholm BB, Chen J-F, Cunha RA, Svenningsson P, Vaugeois J-M (2005) Adenosine and brain function. Int Rev Neurobiol 63(1):191–270
Juhasz J, Sharom FJ, Davis JH (2009) Quantitative characterization of coexisting phases in DOPC/DPPC/cholesterol mixtures: comparing confocal fluorescence microscopy and deuterium nuclear magnetic resonance. Biochim Biophys Acta (BBA)-Biomembr 1788(12):2541–2552
Sichardt K, Nieber K (2007) Adenosine A 1 receptor: functional receptor-receptor interactions in the brain. Purinergic Signal 3(4):285–298
Björklund O, Shang M, Tonazzini I, Daré E, Fredholm BB (2008) Adenosine A1 and A3 receptors protect astrocytes from hypoxic damage. Eur J Pharmacol 596(1-3):6–13
Xie K-q, L-m Z, Cao Y, Zhu J, L-y F (2009) Adenosine A 1 receptor-mediated transactivation of the EGF receptor produces a neuroprotective effect on cortical neurons in vitro. Acta Pharmacol Sin 30(7):889–898
Winerdal M, Winerdal ME, Wang Y-Q, Fredholm BB, Winqvist O, Ådén U (2016) Adenosine A 1 receptors contribute to immune regulation after neonatal hypoxic ischemic brain injury. Purinergic Signal 12(1):89–101
Olsson T, Cronberg T, Rytter A, Asztély F, Fredholm BB, Smith ML, Wieloch T (2004) Deletion of the adenosine A1 receptor gene does not alter neuronal damage following ischaemia in vivo or in vitro. Eur J Neurosci 20(5):1197–1204
Pedata F, Pugliese AM, Coppi E, Dettori I, Maraula G, Cellai L, Melani A (2014) Adenosine receptors modulate acute injury and neuroinflammation in brain ischemia. Mediat Inflamm 2014
Chen J-F, Sonsalla PK, Pedata F, Melani A, Domenici MR, Popoli P, Geiger J, Lopes LV et al (2007) Adenosine A2A receptors and brain injury: broad spectrum of neuroprotection, multifaceted actions and “fine tuning” modulation. Prog Neurobiol 83(5):310–331
Melani A, Pantoni L, Bordoni F, Gianfriddo M, Bianchi L, Vannucchi MG, Bertorelli R, Monopoli A et al (2003) The selective A2A receptor antagonist SCH 58261 reduces striatal transmitter outflow, turning behavior and ischemic brain damage induced by permanent focal ischemia in the rat. Brain Res 959(2):243–250
Melani A, Pugliese AM, Pedata F (2014) Adenosine receptors in cerebral ischemia. In: International Review of Neurobiology, vol 119. Elsevier, pp 309-348
Fredholm BB, Cunha RA, Svenningsson P (2003) Pharmacology of adenosine A2A receptors and therapeutic applications. Curr Top Med Chem 3(4):413–426
Fiebich BL, Biber K, Lieb K, Van Calker D, Berger M, Bauer J, Gebicke-Haerter PJ (1996) Cyclooxygenase-2 expression in rat microglia is induced by adenosine A2a-receptors. Glia 18(2):152–180
Svenningsson P, Le Moine C, Fisone G, Fredholm BB (1999) Distribution, biochemistry and function of striatal adenosine A2A receptors. Prog Neurobiol 59(4):355–396
Yu L, Frith MC, Suzuki Y, Peterfreund RA, Gearan T, Sugano S, Schwarzschild MA, Weng Z et al (2004) Characterization of genomic organization of the adenosine A2A receptor gene by molecular and bioinformatics analyses. Brain Res 1000(1-2):156–173
Trincavelli ML, Melani A, Guidi S, Cuboni S, Cipriani S, Pedata F, Martini C (2008) Regulation of A2A adenosine receptor expression and functioning following permanent focal ischemia in rat brain. J Neurochem 104(2):479–490
Gui L, Duan W, Tian H, Li C, Zhu J, Chen J-F, Zheng J (2009) Adenosine A2A receptor deficiency reduces striatal glutamate outflow and attenuates brain injury induced by transient focal cerebral ischemia in mice. Brain Res 1297:185–193
Mohamed R, Agha A, Abdel-Rahman A, Nassar N (2016) Role of adenosine A2A receptor in cerebral ischemia reperfusion injury: signaling to phosphorylated extracellular signal-regulated protein kinase (pERK1/2). Neuroscience 314:145–159
Mohamed R, Agha A, Nassar N (2012) SCH58261 the selective adenosine A 2A receptor blocker modulates ischemia reperfusion injury following bilateral carotid occlusion: role of inflammatory mediators. Neurochem Res 37(3):538–547
Melani A, Cipriani S, Vannucchi MG, Nosi D, Donati C, Bruni P, Giovannini MG, Pedata F (2009) Selective adenosine A2a receptor antagonism reduces JNK activation in oligodendrocytes after cerebral ischaemia. Brain 132(6):1480–1495
Melani A, Gianfriddo M, Vannucchi MG, Cipriani S, Baraldi PG, Giovannini MG, Pedata F (2006) The selective A2A receptor antagonist SCH 58261 protects from neurological deficit, brain damage and activation of p38 MAPK in rat focal cerebral ischemia. Brain Res 1073:470–480
Melani A, Dettori I, Corti F, Cellai L, Pedata F (2015) Time-course of protection by the selective A 2A receptor antagonist SCH58261 after transient focal cerebral ischemia. Neurol Sci 36(8):1441–1448
Zhou Y, Zeng X, Li G, Yang Q, Xu J, Zhang M, Mao X, Cao Y et al (2019) Inactivation of endothelial adenosine A2A receptors protects mice from cerebral ischaemia-induced brain injury. Br J Pharmacol 176(13):2250–2263
Von Lubitz DK, Lin RC, Jacobson KA (1995) Cerebral ischemia in gerbils: effects of acute and chronic treatment with adenosine A2A receptor agonist and antagonist. Eur J Pharmacol 287(3):295–302
Sheardown MJ, Knutsen LJ (1996) Unexpected neuroprotection observed with the adenosine A2A receptor agonist CGS 21680. Drug Dev Res 39(1):108–114
Melani A, Corti F, Cellai L, Vannucchi MG, Pedata F (2014) Low doses of the selective adenosine A2A receptor agonist CGS21680 are protective in a rat model of transient cerebral ischemia. Brain Res 1551:59–72
Fredholm BB, IJzerman AP, Jacobson KA, Linden J, Müller CE (2011) International Union of Basic and Clinical Pharmacology. LXXXI Nomenclature and classification of adenosine receptors—an update. Pharmacol Rev 63(1):1–34
Dixon AK, Gubitz AK, Sirinathsinghji DJ, Richardson PJ, Freeman TC (1996) Tissue distribution of adenosine receptor mRNAs in the rat. Br J Pharmacol 118(6):1461–1468
Yamagata K, Hakata K, Maeda A, Mochizuki C, Matsufuji H, Chino M, Yamori Y (2007) Adenosine induces expression of glial cell line-derived neurotrophic factor (GDNF) in primary rat astrocytes. Neurosci Res 59(4):467–474
Popoli P, Pepponi R (2012) Potential therapeutic relevance of adenosine A2B and A2A receptors in the central nervous system. CNS Neurol Disord Drug Targets (Form Curr Drug Targets-CNS Neurol Disord) 11(6):664–674
Li Q, Han X, Lan X, Hong X, Li Q, Gao Y, Luo T, Yang Q et al (2017) Inhibition of tPA-induced hemorrhagic transformation involves adenosine A2b receptor activation after cerebral ischemia. Neurobiol Dis 108:173–182
Fusco I, Ugolini F, Lana D, Coppi E, Dettori I, Gaviano L, Nosi D, Cherchi F et al (2018) The selective antagonism of adenosine A2B receptors reduces the synaptic failure and neuronal death induced by oxygen and glucose deprivation in rat CA1 hippocampus in vitro. Front Pharmacol 9:399
Zhou Q-Y, Li C, Olah ME, Johnson RA, Stiles GL, Civelli O (1992) Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc Natl Acad Sci 89(16):7432–7436
Gessi S, Merighi S, Varani K, Leung E, Mac Lennan S, Borea PA (2008) The A3 adenosine receptor: an enigmatic player in cell biology. Pharmacol Ther 117(1):123–140
Lopes LV, Rebola N, Pinheiro PC, Richardson PJ, Oliveira CR, Cunha RA (2003) Adenosine A3 receptors are located in neurons of the rat hippocampus. Neuroreport 14(12):1645–1648
Ji X, Gallorodriguez C, Jacobson KA (1994) A selective agonist affinity label for A3 adenosine receptors. Biochem Biophys Res Commun 203(1):570–576
Cunha RA, Constantino M, Sebastião AM, Ribeiro JA (1995) Modification of A1 and A2a adenosine receptor binding in aged striatum, hippocampus and cortex of the rat. Neuroreport 6(11):1583–1588
Cunha RA, Constantino M, Sebastião A, Johansson B, Fredholm BB (1996) Evidence for high-affinity binding sites for the adenosine A 2A receptor agonist [3 H] CGS 21680 in the rat hippocampus and cerebral cortex that are different from striatal A 2A receptors. Naunyn Schmiedeberg's Arch Pharmacol 353(3):261–271
Latini S, Pedata F (2001) Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem 79(3):463–484
Federova I, Jacobson M, Basile A (2003) Behavioral characterization of mice lacking the A3 receptor: sensitivity to hypoxic degeneration. Cell Mol Neurobiol 23:431–447
Chen GJ, Harvey BK, Shen H, Chou J, Victor A, Wang Y (2006) Activation of adenosine A3 receptors reduces ischemic brain injury in rodents. J Neurosci Res 84(8):1848–1855
Gessi S, Merighi S, Stefanelli A, Fazzi D, Varani K, Borea PA (2013) A1 and A3 adenosine receptors inhibit LPS-induced hypoxia-inducible factor-1 accumulation in murine astrocytes. Pharmacol Res 76:157–170
Choi I-Y, Lee J-C, Ju C, Hwang S, Cho G-S, Lee HW, Choi WJ, Jeong LS et al (2011) A3 adenosine receptor agonist reduces brain ischemic injury and inhibits inflammatory cell migration in rats. Am J Pathol 179(4):2042–2052
Pugliese AM, Coppi E, Spalluto G, Corradetti R, Pedata F (2006) A3 adenosine receptor antagonists delay irreversible synaptic failure caused by oxygen and glucose deprivation in the rat CA1 hippocampus in vitro. Br J Pharmacol 147(5):524–532
Pugliese AM, Latini S, Corradetti R, Pedata F (2003) Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors. Br J Pharmacol 140(2):305–314
Von Lubitz DK, Lin RC-S, Popik P, Carter MF, Jacobson KA (1994) Adenosine A3 receptor stimulation and cerebral ischemia. Eur J Pharmacol 263(1-2):59
Von Lubitz DK, Lin RC-S, Boyd M, Bischofberger N, Jacobson KA (1999) Chronic administration of adenosine A3 receptor agonist and cerebral ischemia: neuronal and glial effects. Eur J Pharmacol 367(2-3):157–163
Pugliese AM, Coppi E, Volpini R, Cristalli G, Corradetti R, Jeong LS, Jacobson KA, Pedata F (2007) Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen–glucose deprivation episodes of different duration. Biochem Pharmacol 74(5):768–779
Van Schaick EA, Jacobson KA, Kim HO, Ijzerman AP, Danhof M (1996) Hemodynamic effects and histamine release elicited by the selective adenosine A3 receptor agonist 2-Cl-IB-MECA in conscious rats. Eur J Pharmacol 308(3):311–314
Doré S (2006) GPCR antagonists as an alternative to COX-2 inhibitors: a case for the PGE2 EP1 receptor. Trends Pharmacol Sci 27(9):458–460
Saleem S, Shah ZA, Urade Y, Doré S (2009) Lipocalin-prostaglandin D synthase is a critical beneficial factor in transient and permanent focal cerebral ischemia. Neuroscience 160(1):248–254
Urade Y, Hayaishi O (2000) Prostaglandin D synthase: structure and function.
Matsuoka T, Hirata M, Tanaka H, Takahashi Y, Murata T, Kabashima K, Sugimoto Y, Kobayashi T, Ushikubi F, Aze Y (2000) Prostaglandin D2 as a mediator of allergic asthma. science 287 (5460):2013-2017
Hata AN, Breyer RM (2004) Pharmacology and signaling of prostaglandin receptors: multiple roles in inflammation and immune modulation. Pharmacol Ther 103(2):147–166
Koch KA, Wessale JL, Moreland R, Reinhart GA, Cox BF (2005) Effects of BW245C, a prostaglandin DP receptor agonist, on systemic and regional haemodynamics in the anaesthetized rat. Clin Exp Pharmacol Physiol 32(11):931–935
Liang X, Wu L, Hand T, Andreasson K (2005) Prostaglandin D2 mediates neuronal protection via the DP1 receptor. J Neurochem 92(3):477–486
Taniguchi H, Mohri I, Okabe-Arahori H, Aritake K, Wada K, Kanekiyo T, Narumiya S, Nakayama M et al (2007) Prostaglandin D2 protects neonatal mouse brain from hypoxic ischemic injury. J Neurosci 27(16):4303–4312
Ahmad AS, Ahmad M, Maruyama T, Narumiya S, Doré S (2010) Prostaglandin D 2 DP1 receptor is beneficial in ischemic stroke and in acute exicitotoxicity in young and old mice. Age 32(3):271–282
Schuligoi R, Schmidt R, Geisslinger G, Kollroser M, Peskar BA, Heinemann A (2007) PGD2 metabolism in plasma: kinetics and relationship with bioactivity on DP1 and CRTH2 receptors. Biochem Pharmacol 74(1):107–117
Ahmad A (2014) PGD2 DP1 receptor stimulation following stroke ameliorates cerebral blood flow and outcomes. Neuroscience 279:260–268
Sawyer N, Cauchon E, Chateauneuf A, Cruz RP, Nicholson DW, Metters KM, O’Neill GP, Gervais FG (2002) Molecular pharmacology of the human prostaglandin D2 receptor, CRTH2. Br J Pharmacol 137(8):1163–1172
Bonfill-Teixidor E, Otxoa-de-Amezaga A, Font-Nieves M, Sans-Fons MG, Planas AM (2017) Differential expression of E-type prostanoid receptors 2 and 4 in microglia stimulated with lipopolysaccharide. J Neuroinflammation 14(1):3
Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S et al (2006) Prostaglandin E 2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nat Med 12(2):225–229
McCullough L, Wu L, Haughey N, Liang X, Hand T, Wang Q, Breyer RM, Andreasson K (2004) Neuroprotective function of the PGE2 EP2 receptor in cerebral ischemia. J Neurosci 24(1):257–268
Echeverria V, Clerman A, Doré S (2005) Stimulation of PGE2 receptors EP2 and EP4 protects cultured neurons against oxidative stress and cell death following β-amyloid exposure. Eur J Neurosci 22(9):2199–2206
Shimamura M, Zhou P, Casolla B, Qian L, Capone C, Kurinami H, ladecola C, Anrather J (2013) Prostaglandin E2 type 1 receptors contribute to neuronal apoptosis after transient forebrain ischemia. J Cereb Blood Flow Metab 33 (8):1207-1214
Ahmad AS, Kim YT, Ahmad M, Maruyama T, Doré S (2008) Selective blockade of PGE 2 EP1 receptor protects brain against experimental ischemia and excitotoxicity, and hippocampal slice cultures against oxygen-glucose deprivation. Neurotox Res 14(4):343–351
Zhou P, Qian L, Chou T, Iadecola C (2008) Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis 29(3):543–551
Frankowski JC, DeMars KM, Ahmad AS, Hawkins KE, Yang C, Leclerc JL, Doré S, Candelario-Jalil E (2015) Detrimental role of the EP1 prostanoid receptor in blood-brain barrier damage following experimental ischemic stroke. Sci Rep 5:17956
Saleem S, Rc L, Wei G, Doré S (2007) Effects of EP1 receptor on cerebral blood flow in the middle cerebral artery occlusion model of stroke in mice. J Neurosci Res 85(11):2433–2440
Ikeda-Matsuo Y, Tanji H, Narumiya S, Sasaki Y (2011) Inhibition of prostaglandin E2 EP3 receptors improves stroke injury via anti-inflammatory and anti-apoptotic mechanisms. J Neuroimmunol 238(1-2):34–43
Saleem S, Kim YT, Maruyama T, Narumiya S, Doré S (2009) Reduced acute brain injury in PGE2 EP3 receptor-deficient mice after cerebral ischemia. J Neuroimmunol 208(1-2):87–93
Leclerc JL, Lampert AS, Diller MA, Doré S (2015) Genetic deletion of the pge2 ep3 receptor improves anatomical and functional outcomes after intracerebral hemorrhage. Eur J Neurosci 41(10):1381
Ahmad M, Ahmad AS, Zhuang H, Maruyama T, Narumiya S, Doré S (2007) Stimulation of prostaglandin E2-EP3 receptors exacerbates stroke and excitotoxic injury. J Neuroimmunol 184(1-2):172–179
Jadhav V, Jabre A, Lin S-Z, Lee TJ-F (2004) EP1-and EP3-receptors mediate prostaglandin E2–induced constriction of porcine large cerebral arteries. J Cereb Blood Flow Metab 24(12):1305–1316
Bilak M, Wu L, Wang Q, Haughey N, Conant K, St. Hillaire C, Andreasson K (2004) PGE2 receptors rescue motor neurons in a model of amyotrophic lateral sclerosis. Ann Neurol 56(2):240–248
Martin M, Meyer-Kirchrath J, Kaber G, Jacoby C, Flögel U, Jr S, Rüther U, Schrör K et al (2005) Clinical perspective. Circulation 112(3):400–406
Liu D, Wu L, Breyer R, Mattson MP, Andreasson K (2005) Neuroprotection by the PGE2 EP2 receptor in permanent focal cerebral ischemia. Ann Neurol 57(5):758–761
Takadera T, Ohyashiki T (2006) Prostaglandin E2 deteriorates N-methyl-D-aspartate receptor-mediated cytotoxicity possibly by activating EP2 receptors in cultured cortical neurons. Life Sci 78(16):1878–1883
Takadera T, Shiraishi Y, Ohyashiki T (2004) Prostaglandin E2 induced caspase-dependent apoptosis possibly through activation of EP2 receptors in cultured hippocampal neurons. Neurochem Int 45(5):713–719
Liang X, Lin L, Woodling NS, Wang Q, Anacker C, Pan T, Merchant M, Andreasson K (2011) Signaling via the prostaglandin E 2 receptor EP4 exerts neuronal and vascular protection in a mouse model of cerebral ischemia. J Clin Invest 121(11):4362–4371
Ahmad AS, Ahmad M, de Brum-Fernandes AJ, Doré S (2005) Prostaglandin EP4 receptor agonist protects against acute neurotoxicity. Brain Res 1066(1-2):71–77
DeMars KM, McCrea AO, Siwarski DM, Sanz BD, Yang C, Candelario-Jalil E (2018) Protective effects of L-902,688, a prostanoid EP4 receptor agonist, against acute blood-brain barrier damage in experimental ischemic stroke. Front Neurosci 12:89
Chemtob S, Beharry K, Rex J, Varma DR, Aranda JV (1990) Prostanoids determine the range of cerebral blood flow autoregulation of newborn piglets. Stroke 21(5):777–784
Iijima T, Iwasaki H, Narumiya S, Ushikubi F (2005) Takayama K, Yuhki K, Ono K, Fujino T, Hara A, Yamada T, Kuriyama S, Karibe H, Okada Y, Takahata O, Taniguchi T. Nat Med 11(5):562–566
J-i K, Hasimoto H, Aino H, Gotoh M, Baba A, Sugimoto Y, Negishi M, Ichikawa A (1994) Cloning and expression of a cDNA for rat prostaglandin F2α receptor. Prostaglandins 48(1):31–41
Snetkov VA, Knock GA, Baxter L, Thomas GD, Ward JP, Aaronson PI (2006) Mechanisms of the prostaglandin F2α-induced rise in [Ca2+] i in rat intrapulmonary arteries. J Physiol 571(1):147–163
Saleem S, Ahmad AS, Maruyama T, Narumiya S, Doré S (2009) PGF 2α FP receptor contributes to brain damage following transient focal brain ischemia. Neurotox Res 15(1):62–70
Kim YT, Moon SK, Maruyama T, Narumiya S, Doré S (2012) Prostaglandin FP receptor inhibitor reduces ischemic brain damage and neurotoxicity. Neurobiol Dis 48(1):58–65
Fang Y-C, Wu J-S, Chen J-J, Cheung W-M, Tseng P-H, Tarn K-B, Shyue S-K, Chen J-J et al (2006) Induction of prostacyclin/PGI2 synthase expression after cerebral ischemia–reperfusion. J Cereb Blood Flow Metab 26(4):491–501
Santhanam AVR, Smith LA, Katusic ZS (2010) Brain-derived neurotrophic factor stimulates production of prostacyclin in cerebral arteries. Stroke 41(2):350–356
Matsuda S, Wen T-C, Karasawa Y, Araki H, Otsuka H, Ishihara K, Sakanaka M (1997) Protective effect of a prostaglandin I2 analog, TEI-7165, on ischemic neuronal damage in gerbils. Brain Res 769(2):321–328
Breyer RM, Bagdassarian CK, Myers SA, Breyer MD (2001) Prostanoid receptors: subtypes and signaling. Annu Rev Pharmacol Toxicol 41(1):661–690
Wright DH, Abran D, Bhattacharya M, Hou X, Bernier SG, Bouayad A, Fouron J-C, Vazquez-Tello A et al (2001) Prostanoid receptors: ontogeny and implications in vascular physiology. Am J Phys Regul Integr Comp Phys 281(5):R1343–R1360
Wei G, Kibler K, Koehler RC, Maruyama T, Narumiya S, Doré S (2008) Prostacyclin receptor deletion aggravates hippocampal neuronal loss after bilateral common carotid artery occlusion in mouse. Neuroscience 156(4):1111–1117
Yang C, DeMars KM, Alexander JC, Febo M, Candelario-Jalil E (2017) Sustained neurological recovery after stroke in aged rats treated with a novel prostacyclin analog. Stroke 48(7):1948–1956
Saleem S, Shah Z, Maruyama T, Narumiya S, Dore S (2010) Neuroprotective properties of prostaglandin I2 IP receptor in focal cerebral ischemia. Neuroscience 170(1):317–323
Shakil H, Saleem S (2014) Prostaglandin i2 ip receptor agonist, beraprost, prevents transient global cerebral ischemia induced hippocampal ca1 injury in aging mice. Journal of neurological disorders 2
Hamburg M, Svensson J, Samuelsson B (1975) Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A 72:2994–2998
Nakahata N (2008) Thromboxane A2: physiology/pathophysiology, cellular signal transduction and pharmacology. Pharmacol Ther 118(1):18–35
Fisher M (1993) Increased thromboxane biosynthesis in patients with acute cerebral ischemia. Stroke 24(6):912–912
GIULIAN D, CORPUZ M, RICHMOND B, WENDT E, HALL ER (1996) Activated microglia are the principal glial source of thromboxane in the central nervous system. Neurochem Int 29(1):65–76
Hirata M (1991) Hayashi Y. Ushikubi F, Yokota Y, Kageyama R, Nakanishi S, Narumiya S. Cloning and expression of cDNA for a human thromboxane A2 receptor. Nature 349:617–620
Bevan S (1996) Intracellular messengers and signal transduction in nociceptors. Neurobiology of nociceptors
J-i K, Hashimoto H, Sugimoto Y, Sawada M, Negishi M, Suzumura A, Marunouchi T, Ichikawa A et al (1995) cDNA cloning of a thromboxane A2 receptor from rat astrocytes. Biochim Biophys Acta (BBA)-Mol Cell Res 1265(2-3):220–223
Nakahata N, Miyamoto A, Ohkubo S, Ishimoto H, Sakai K, Nakanishi H, Ohshika H, Ohizumi Y (1995) Gq/11 communicates with thromboxane A2 receptors in human astrocytoma cells, rabbit astrocytes and human platelets. Res Commun Mol Pathol Pharmacol 87(3):243
Blackman SC, Dawson G, Antonakis K, Le Breton GC (1998) The identification and characterization of oligodendrocyte thromboxane A2 receptors. J Biol Chem 273(1):475–483
Yan A, Zhang T, Yang X, Shao J, Fu N, Shen F, Fu Y, Xia W (2016) Thromboxane A2 receptor antagonist SQ29548 reduces ischemic stroke-induced microglia/macrophages activation and enrichment, and ameliorates brain injury. Sci Rep 6:35885
Huang J, Ramamurthy SK, Lin X, Le Breton GC (2004) Cell Signalling through thromboxane A. 2:521-533
Yang W, Yan A, Zhang T, Shao J, Liu T, Yang X, Xia W, Fu Y (2016) Thromboxane A2 receptor stimulation enhances microglial interleukin-1β and NO biosynthesis mediated by the activation of ERK pathway. Front Aging Neurosci 8:8
Wootten D, Christopoulos A, Marti-Solano M, Babu MM, Sexton PM (2018) Mechanisms of signalling and biased agonism in G protein-coupled receptors. Nat Rev Mol Cell Biol 19(10):638–653
Seyedabadi M, Ghahremani MH, Albert PR (2019) Biased signaling of G protein coupled receptors (GPCRs): molecular determinants of GPCR/transducer selectivity and therapeutic potential. Pharmacol Ther 200:148–178
Davenport AP, Scully CC, de Graaf C, Brown AJ, Maguire JJ (2020) Advances in therapeutic peptides targeting G protein-coupled receptors. Nat Rev Drug Discov 19(6):389–413
YAMAGISHI K, Iso H, Kitamura A, Sankai T, Tanigawa T, Naito Y, Sato S, Imano H et al (2003) Smoking raises the risk of total and ischemic strokes in hypertensive men. Hypertens Res 26(3):209–217
Nejati M, Atlasi MA, Karimian M, Nikzad H, Tameh AA (2018) Lipoprotein lipase gene polymorphisms as risk factors for stroke: a computational and meta-analysis. Iran J Basic Med Sci 21(7):701
Hsieh C, Bowcock A, Farrer L, Hebert J, Huang K, Cavalli-Sforza L, Julius D, Francke U (1990) The serotonin receptor subtype 2 locusHTR2 is on human chromosome 13 near genes for esterase D and retinoblastoma-1 and on mouse chromosome 14. Somat Cell Mol Genet 16(6):567–574
Kusumi I, Suzuki K, Sasaki Y, Kameda K, Sasaki T, Koyama T (2002) Serotonin 5-HT2A receptor gene polymorphism, 5-HT2A receptor function and personality traits in healthy subjects: a negative study. J Affect Disord 68(2-3):235–241
Polesskaya OO, Sokolov BP (2002) Differential expression of the “C” and “T” alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J Neurosci Res 67(6):812–822
Olesen OF, Bennike B, Dam H, Mellerup E (2006) Association of the 5-HT2A receptor gene polymorphism 102 T/C with ischemic stroke. J Mol Neurosci 30(3):323–328
Kim J-M, Stewart R, Bae K-Y, Kim S-W, Kang H-J, Shin I-S, Kim J-T, Park M-S et al (2012) Serotonergic and BDNF genes and risk of depression after stroke. J Affect Disord 136(3):833–840
Amberger JS, Bocchini CA, Schiettecatte F, Scott AF, Hamosh A (2015) OMIM. org: Online Mendelian Inheritance in Man (OMIM®), an online catalog of human genes and genetic disorders. Nucleic Acids Res 43(D1):D789–D798
Yildirim BO, Derksen JJ (2013) Systematic review, structural analysis, and new theoretical perspectives on the role of serotonin and associated genes in the etiology of psychopathy and sociopathy. Neurosci Biobehav Rev 37(7):1254–1296
Iwamoto K, Bundo M, Kato T (2009) Serotonin receptor 2C and mental disorders: genetic, expression, and RNA editing studies. RNA Biol 6(3):248–253
Tang W, Tang N, Liao C, Liang H, Mok V, Ungvari G, Wong K (2013) Serotonin receptor 2C gene polymorphism associated with post-stroke depression in Chinese patients. Genet Mol Res 12(2):1546–1553
Doll BB, Hutchison KE, Frank MJ (2011) Dopaminergic genes predict individual differences in susceptibility to confirmation bias. J Neurosci 31(16):6188–6198
Kim B-R, Kim HY, Chun YI, Yun Y-M, Kim H, Choi D-H, Lee J (2016) Association between genetic variation in the dopamine system and motor recovery after stroke. Restor Neurol Neurosci 34(6):925–934
Di Renzo G, Pignataro G, Annunziato L (2009) Why have ionotropic and metabotropic glutamate antagonists failed in stroke therapy? New strategies in stroke intervention. Springer, In, pp. 13–25
Robinson JE, Wolfe SM, Kaiser-Rogers K, Greenwood RS (2016) Stroke-like presentation following febrile seizure in a patient with 1q43q44 deletion syndrome. Front Neurol 7:67
Mazdeh M, Noroozi R, Komaki A, Azari I, Ghafouri-Fard S, Taheri M (2019) A single nucleotide polymorphism in the metabotropic glutamate receptor 7 gene is associated with multiple sclerosis in Iranian population. Mult Scler Relat Disord 28:189–192
Safari MR, Beirami AD, Khazaie M, Komaki A, Noroozi R, Ghafouri-Fard S, Taheri M (2020) GRM7 polymorphisms are not associated with ischemic stroke in Iranian population. Nucleosides Nucleotides Nucleic Acids 39(5):792–798
Bastien L, Sawyer N, Grygorczyk R, Metters KM, Adam M (1994) Cloning, functional expression, and characterization of the human prostaglandin E2 receptor EP2 subtype. J Biol Chem 269(16):11873–11877
Jinnai N, Sakagami T, Sekigawa T, Kakihara M, Nakajima T, Yoshida K, Goto S, Hasegawa T et al (2004) Polymorphisms in the prostaglandin E2 receptor subtype 2 gene confer susceptibility to aspirin-intolerant asthma: a candidate gene approach. Hum Mol Genet 13(24):3203–3217
Hegener H, Diehl K, Kurth T, Gaziano J, Ridker P, Zee R (2006) Polymorphisms of prostaglandin-endoperoxide synthase 2 gene, and prostaglandin-E receptor 2 gene, C-reactive protein concentrations and risk of atherothrombosis: a nested case–control approach. J Thromb Haemost 4(8):1718–1722
Ogawa Y, Tanaka I, Inoue M, Yoshitake Y, Isse N, Nakagawa O, Usui T, Itoh H et al (1995) Structural organization and chromosomal assignment of the human prostacyclin receptor gene. Genomics 27(1):142–148
Stitham J, Stojanovic A, Hwa J (2002) Impaired receptor binding and activation associated with a human prostacyclin receptor polymorphism. J Biol Chem 277(18):15439–15444
Shimizu M, Yoshimura S, Takizawa S, Kohara S, Inoko H, Takagi S (2013) Effect of single nucleotide polymorphisms of the prostacyclin receptor gene on platelet activation in Japanese healthy subjects and patients with cerebral infarction. J Clin Neurosci 20(6):851–856
Nüsing RM, Hirata M, Kakizuka A, Eki T, Ozawa K, Narumiya S (1993) Characterization and chromosomal mapping of the human thromboxane A2 receptor gene. J Biol Chem 268(33):25253–25259
Zhao J, Zheng L, Fei Q, Fu Y, Weng Y, Wu H, Li H, Jun Q et al (2013) Association of thromboxane A2 receptor gene polymorphisms with cerebral infarction in a Chinese population. Neurol Sci 34(10):1791–1796
Kaneko Y, Nakayama T, Saito K, Morita A, Sato I, Maruyama A, Soma M, Takahashi T et al (2006) Relationship between the thromboxane A 2 receptor gene and susceptibility to cerebral infarction. Hypertens Res 29(9):665–671
Park SA, Park BL, Park JH, Lee TK, Sung KB, Lee YK, Chang HS, Park C-S et al (2009) Association of polymorphisms in thromboxane A2 receptor and thromboxane A synthase 1 with cerebral infarction in a Korean population. BMB Rep 42(4):200–205
Acknowledgements
We thank all the people who consult us in this work.
Funding
This work was supported by a grant from Kashan University of Medical Sciences (grant no. 92071).
Author information
Authors and Affiliations
Contributions
MK and AAT designed and supervised this work. ZV provided the draft of the manuscript. MTJ and CB initially revised the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Ethical Approval
The article does not contain any studies with animals or human subjects.
Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vahidinia, Z., Joghataei, M.T., Beyer, C. et al. G-Protein-Coupled Receptors and Ischemic Stroke: a Focus on Molecular Function and Therapeutic Potential. Mol Neurobiol 58, 4588–4614 (2021). https://doi.org/10.1007/s12035-021-02435-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02435-5