Abstract
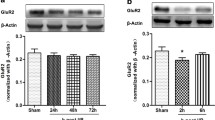
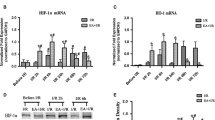
Electroacupuncture (EA) pretreatment elicits the neuroprotective effect against cerebral ischemic injury through cannabinoid receptor type 1 receptor (CB1R). In current study, we aimed to investigate whether the signal transducer and activator of transcription 3 (STAT3) and manganese superoxide dismutase (Mn-SOD) were involved in the antioxidant effect of EA pretreatment through CB1R. At 2 h after EA pretreatment, focal cerebral ischemic injury was induced by transient middle cerebral artery occlusion for 60 min in C57BL/6 mice. The expression of Mn-SOD in the penumbra was assessed by Western blot and immunoflourescent staining at 2 h after reperfusion. In the presence or absence of Mn-SOD small interfering RNA (siRNA), the neurological deficit score, the infarct volume, the terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling (TUNEL) staining, and oxidative stress were evaluated. Furthermore, the Mn-SOD protein expression and phosphorylation of STAT3 at Y705 were also determined in the presence and absence of CB1R antagonists (AM251, SR141716) and CB1R agonists (arachidonyl-2-chloroethylamide (ACEA), WIN 55,212-2). EA pretreatment upregulated the Mn-SOD protein expression and Mn-SOD-positive neuronal cells at 2 h after reperfusion. EA pretreatment also attenuated oxidative stress, inhibited cellular apoptosis, and induced neuroprotection against ischemic damage, whereas these beneficial effects of EA pretreatment were reversed by knockdown of Mn-SOD. Mn-SOD upregulation and STAT3 phosphorylation by EA pretreatment were abolished by two CB1R antagonists, while pretreatment with two CB1R agonists increased the expression of Mn-SOD and phosphorylation level of STAT3. Mn-SOD upregulation by EA attenuates ischemic oxidative damage through CB1R-mediated STAT3 phosphorylation in stroke mice, which may represent one new mechanism of EA pretreatment-induced neuroprotection against cerebral ischemia.






Similar content being viewed by others
Abbreviations
- t-PA:
-
Tissue-type plasminogen activators
- ROS:
-
Reactive oxygen species
- I/R:
-
Ischemia/reperfusion
- SOD:
-
Superoxide dismutase
- Mn-SOD:
-
Manganese superoxide dismutase
- Cu/Zn-SOD:
-
Cu/Zn superoxide dismutase
- EA:
-
Electroacupuncture
- MCAO:
-
Middle cerebral artery occlusion
- 2-AG:
-
2-Arachidonylglycerol
- AEA:
-
N-Arach-idonoylethanolamine-anandamide
- CB1R:
-
Cannabinoid receptor type 1 receptor
- Trx:
-
Thioredoxin
- GSH:
-
Glutathione
- MDA:
-
Malondialdehyde
- STAT3:
-
Signal transducer and activator of transcription 3
- TUNEL:
-
Terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling
References
Yang G, Wang Y, Zeng Y, Gao GF, Liang X, Zhou M, Wan X, Yu S, Jiang Y, Naghavi M, Vos T, Wang H, Lopez AD, Murray CJ (2013) Rapid health transition in china, 1990–2010: findings from the global burden of disease study 2010. Lancet 381(9882):1987–2015. doi:10.1016/S0140-6736(13)61097-1
Fonarow GC, Smith EE, Saver JL, Reeves MJ, Bhatt DL, Grau-Sepulveda MV, Olson DM, Hernandez AF, Peterson ED, Schwamm LH (2011) Timeliness of tissue-type plasminogen activator therapy in acute ischemic stroke: patient characteristics, hospital factors, and outcomes associated with door-to-needle times within 60 minutes. Circulation 123(7):750–758. doi:10.1161/CIRCULATIONAHA.110.974675
McCord JM (1985) Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med 312(3):159–163. doi:10.1056/NEJM198501173120305
Guerra-Araiza C, Alvarez-Mejia AL, Sanchez-Torres S, Farfan-Garcia E, Mondragon-Lozano R, Pinto-Almazan R, Salgado-Ceballos H (2013) Effect of natural exogenous antioxidants on aging and on neurodegenerative diseases. Free Radic Res 47(6–7):451–462. doi:10.3109/10715762.2013.795649
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12. doi:10.1016/j.freeradbiomed.2013.05.027
Jung JE, Kim GS, Chen H, Maier CM, Narasimhan P, Song YS, Niizuma K, Katsu M, Okami N, Yoshioka H, Sakata H, Goeders CE, Chan PH (2010) Reperfusion and neurovascular dysfunction in stroke: from basic mechanisms to potential strategies for neuroprotection. Mol Neurobiol 41(2–3):172–179. doi:10.1007/s12035-010-8102-z
Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH (1998) Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci 18(1):205–213
Fujimura M, Morita-Fujimura Y, Kawase M, Copin JC, Calagui B, Epstein CJ, Chan PH (1999) Manganese superoxide dismutase mediates the early release of mitochondrial cytochrome C and subsequent DNA fragmentation after permanent focal cerebral ischemia in mice. J Neurosci 19(9):3414–3422
Fukui M, Zhu BT (2010) Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic Biol Med 48(6):821–830. doi:10.1016/j.freeradbiomed.2009.12.024
Fukui M, Choi HJ, Zhu BT (2010) Mechanism for the protective effect of resveratrol against oxidative stress-induced neuronal death. Free Radic Biol Med 49(5):800–813. doi:10.1016/j.freeradbiomed.2010.06.002
Madhavan L, Ourednik V, Ourednik J (2008) Neural stem/progenitor cells initiate the formation of cellular networks that provide neuroprotection by growth factor-modulated antioxidant expression. Stem Cells 26(1):254–265. doi:10.1634/stemcells. 2007-0221
Park JH, Joo HS, Yoo KY, Shin BN, Kim IH, Lee CH, Choi JH, Byun K, Lee B, Lim SS, Kim MJ, Won MH (2011) Extract from Terminalia chebula seeds protect against experimental ischemic neuronal damage via maintaining SODs and BDNF levels. Neurochem Res 36(11):2043–2050. doi:10.1007/s11064-011-0528-9
Kitagawa K (2012) Ischemic tolerance in the brain: endogenous adaptive machinery against ischemic stress. J Neurosci Res 90(5):1043–1054. doi:10.1002/jnr.23005
Yang Q, Yan W, Li X, Hou L, Dong H, Wang Q, Dong H, Wang S, Zhang X, Xiong L (2012) Activation of canonical notch signaling pathway is involved in the ischemic tolerance induced by sevoflurane preconditioning in mice. Anesthesiology 117(5):996–1005. doi:10.1097/ALN.0b013e31826cb469
Nie H, Xiong L, Lao N, Chen S, Xu N, Zhu Z (2006) Hyperbaric oxygen preconditioning induces tolerance against spinal cord ischemia by upregulation of antioxidant enzymes in rabbits. J Cereb Blood Flow Metab 26(5):666–674. doi:10.1038/sj.jcbfm.9600221
Xiong L, Lu Z, Hou L, Zheng H, Zhu Z, Wang Q, Chen S (2003) Pretreatment with repeated electroacupuncture attenuates transient focal cerebral ischemic injury in rats. Chin Med J 116(1):108–111
Wang Q, Xiong L, Chen S, Liu Y, Zhu X (2005) Rapid tolerance to focal cerebral ischemia in rats is induced by preconditioning with electroacupuncture: window of protection and the role of adenosine. Neurosci Lett 381(1–2):158–162. doi:10.1016/j.neulet.2005.02.019
Wang Q, Peng Y, Chen S, Gou X, Hu B, Du J, Lu Y, Xiong L (2009) Pretreatment with electroacupuncture induces rapid tolerance to focal cerebral ischemia through regulation of endocannabinoid system. Stroke 40(6):2157–2164. doi:10.1161/STROKEAHA.108.541490
Leker RR, Shohami E, Abramsky O, Ovadia H (1999) Dexanabinol; a novel neuroprotective drug in experimental focal cerebral ischemia. J Neurol Sci 162(2):114–119
Kim SH, Won SJ, Mao XO, Jin K, Greenberg DA (2005) Involvement of protein kinase A in cannabinoid receptor-mediated protection from oxidative neuronal injury. J Pharmacol Exp Ther 313(1):88–94. doi:10.1124/jpet.104.079509
Siu FK, Lo SC, Leung MC (2005) Electro-acupuncture potentiates the disulphide-reducing activities of thioredoxin system by increasing thioredoxin expression in ischemia-reperfused rat brains. Life Sci 77(4):386–399. doi:10.1016/j.lfs.2004.10.069
Liu ZB, Niu WM (2007) Protective effect of electroacupuncture of “xiusanzhen” on neuronal mitochondria in rats with acute cerebral ischemia-reperfusion injury. Zhen Ci Yan Jiu 32(3):163–166
Zhou H, Zhang Z, Wei H, Wang F, Guo F, Gao Z, Marsicano G, Wang Q, Xiong L (2013) Activation of STAT3 is involved in neuroprotection by electroacupuncture pretreatment via cannabinoid CB1 receptors in rats. Brain Res 1529:154–164. doi:10.1016/j.brainres.2013.07.006
Jung JE, Kim GS, Narasimhan P, Song YS, Chan PH (2009) Regulation of Mn-superoxide dismutase activity and neuroprotection by STAT3 in mice after cerebral ischemia. J Neurosci 29(21):7003–7014. doi:10.1523/JNEUROSCI. 1110-09.2009
Wang Q, Li X, Chen Y, Wang F, Yang Q, Chen S, Min Y, Li X, Xiong L (2011) Activation of epsilon protein kinase C-mediated anti-apoptosis is involved in rapid tolerance induced by electroacupuncture pretreatment through cannabinoid receptor type 1. Stroke 42(2):389–396. doi:10.1161/STROKEAHA.110.597336
Zoppi S, Perez Nievas BG, Madrigal JL, Manzanares J, Leza JC, Garcia-Bueno B (2011) Regulatory role of cannabinoid receptor 1 in stress-induced excitotoxicity and neuroinflammation. Neuropsychopharmacology 36(4):805–818. doi:10.1038/npp.2010.214
Cai M, Ma YL, Qin P, Li Y, Zhang LX, Nie H, Peng Z, Dong H, Dong HL, Hou WG, Xiong LZ (2014) The loss of estrogen efficacy against cerebral ischemia in aged postmenopausal female mice. Neurosci Lett 558:115–119. doi:10.1016/j.neulet.2013.11.007
Garcia JH, Wagner S, Liu KF, Hu XJ (1995) Neurological deficit and extent of neuronal necrosis attributable to middle cerebral artery occlusion in rats. Statistical validation. Stroke 26(4):627–634, discussion 635
Wang Q, Wang F, Li X, Yang Q, Li X, Xu N, Huang Y, Zhang Q, Gou X, Chen S, Xiong L (2012) Electroacupuncture pretreatment attenuates cerebral ischemic injury through alpha7 nicotinic acetylcholine receptor-mediated inhibition of high-mobility group box 1 release in rats. J Neuroinflammation 9:24. doi:10.1186/1742-2094-9-24
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Huttemann M (2013) Molecular mechanisms of ischemia-reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23. doi:10.1007/s12035-012-8344-z
Chen H, Yoshioka H, Kim GS, Jung JE, Okami N, Sakata H, Maier CM, Narasimhan P, Goeders CE, Chan PH (2011) Oxidative stress in ischemic brain damage: mechanisms of cell death and potential molecular targets for neuroprotection. Antioxid Redox Signal 14(8):1505–1517. doi:10.1089/ars.2010.3576
Dong H, Fan YH, Zhang W, Wang Q, Yang QZ, Xiong LZ (2009) Repeated electroacupuncture preconditioning attenuates matrix metalloproteinase-9 expression and activity after focal cerebral ischemia in rats. Neurol Res 31(8):853–858. doi:10.1179/174313209X393960
Mukhopadhyay P, Rajesh M, Pan H, Patel V, Mukhopadhyay B, Batkai S, Gao B, Hasko G, Pacher P (2010) Cannabinoid-2 receptor limits inflammation, oxidative/nitrosative stress, and cell death in nephropathy. Free Radic Biol Med 48(3):457–467. doi:10.1016/j.freeradbiomed.2009.11.022
Horvath B, Magid L, Mukhopadhyay P, Batkai S, Rajesh M, Park O, Tanchian G, Gao RY, Goodfellow CE, Glass M, Mechoulam R, Pacher P (2012) A new cannabinoid CB2 receptor agonist HU-910 attenuates oxidative stress, inflammation and cell death associated with hepatic ischaemia/reperfusion injury. Br J Pharmacol 165(8):2462–2478. doi:10.1111/j.1476-5381.2011.01381.x
Chen Y, Buck J (2000) Cannabinoids protect cells from oxidative cell death: a receptor-independent mechanism. J Pharmacol Exp Ther 293(3):807–812
Marsicano G, Moosmann B, Hermann H, Lutz B, Behl C (2002) Neuroprotective properties of cannabinoids against oxidative stress: role of the cannabinoid receptor CB1. J Neurochem 80(3):448–456
Mukhopadhyay P, Rajesh M, Batkai S, Patel V, Kashiwaya Y, Liaudet L, Evgenov OV, Mackie K, Hasko G, Pacher P (2010) CB1 cannabinoid receptors promote oxidative stress and cell death in murine models of doxorubicin-induced cardiomyopathy and in human cardiomyocytes. Cardiovasc Res 85(4):773–784. doi:10.1093/cvr/cvp369
Rajesh M, Batkai S, Kechrid M, Mukhopadhyay P, Lee WS, Horvath B, Holovac E, Cinar R, Liaudet L, Mackie K, Hasko G, Pacher P (2012) Cannabinoid 1 receptor promotes cardiac dysfunction, oxidative stress, inflammation, and fibrosis in diabetic cardiomyopathy. Diabetes 61(3):716–727. doi:10.2337/db11-0477
Horvath B, Mukhopadhyay P, Hasko G, Pacher P (2012) The endocannabinoid system and plant-derived cannabinoids in diabetes and diabetic complications. Am J Pathol 180(2):432–442. doi:10.1016/j.ajpath.2011.11.003
Jin KL, Mao XO, Goldsmith PC, Greenberg DA (2000) CB1 cannabinoid receptor induction in experimental stroke. Ann Neurol 48(2):257–261
Parmentier-Batteur S, Jin K, Mao XO, Xie L, Greenberg DA (2002) Increased severity of stroke in CB1 cannabinoid receptor knock-out mice. J Neurosci 22(22):9771–9775
Mishima K, Hayakawa K, Abe K, Ikeda T, Egashira N, Iwasaki K, Fujiwara M (2005) Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism. Stroke 36(5):1077–1082. doi:10.1161/01.STR.0000163083.59201.34
Spiegelman BM (2007) Transcriptional control of mitochondrial energy metabolism through the PGC1 coactivators. Novartis Found Symp 287:60–63, discussion 63–69
Kiningham KK, Xu Y, Daosukho C, Popova B, St Clair DK (2001) Nuclear factor kappaB-dependent mechanisms coordinate the synergistic effect of PMA and cytokines on the induction of superoxide dismutase 2. Biochem J 353(Pt 1):147–156
Murley JS, Baker KL, Miller RC, Darga TE, Weichselbaum RR, Grdina DJ (2011) SOD2-mediated adaptive responses induced by low-dose ionizing radiation via TNF signaling and amifostine. Free Radic Biol Med 51(10):1918–1925. doi:10.1016/j.freeradbiomed.2011.08.032
Yang Y, Duan W, Jin Z, Yi W, Yan J, Zhang S, Wang N, Liang Z, Li Y, Chen W, Yi D, Yu S (2013) JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res 55(3):275–286. doi:10.1111/jpi.12070
Dixit D, Sharma V, Ghosh S, Koul N, Mishra PK, Sen E (2009) Manumycin inhibits STAT3, telomerase activity, and growth of glioma cells by elevating intracellular reactive oxygen species generation. Free Radic Biol Med 47(4):364–374. doi:10.1016/j.freeradbiomed.2009.04.031
Terui K, Enosawa S, Haga S, Zhang HQ, Kuroda H, Kouchi K, Matsunaga T, Yoshida H, Engelhardt JF, Irani K, Ohnuma N, Ozaki M (2004) Stat3 confers resistance against hypoxia/reoxygenation-induced oxidative injury in hepatocytes through upregulation of Mn-SOD. J Hepatol 41(6):957–965. doi:10.1016/j.jhep.2004.08.019
Acknowledgments and Funding
This work was supported by the National Natural Science Foundation of China (Grant 81072888 and 81173394), the Overseas, Hong Kong & Macao Scholars Collaborated Researching Fund (Grant 81228022), and the National Key Basic Research and Development Program (973, Grant 2014 CB543202).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Sisi Sun and Xiyao Chen contributed to this work equally.
Rights and permissions
About this article
Cite this article
Sun, S., Chen, X., Gao, Y. et al. Mn-SOD Upregulation by Electroacupuncture Attenuates Ischemic Oxidative Damage via CB1R-Mediated STAT3 Phosphorylation. Mol Neurobiol 53, 331–343 (2016). https://doi.org/10.1007/s12035-014-8971-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8971-7