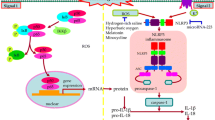
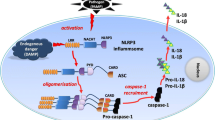
Abstract
Intracerebral hemorrhage (ICH) is the most fatal subtype of stroke; there is still a lack of effective treatment. Microglia are a major component of the innate immune system, and they respond to acute brain injury by activating and forming classic M1-like (pro-inflammatory) or alternative M2-like (anti-inflammatory) phenotype. The existence of the polarization indicates that the role of microglia in disease’s progression and recovery after ICH is still unclear, perhaps involving microglial secretion of anti-inflammatory or pro-inflammatory cytokines and chemokines. The NOD-like receptor family, pyrin domain-containing 3 (NLRP3) inflammasome is considered to be the main participant in neuroinflammation. Recent evidence has shown that NLRP3 inflammasome can be activated after ICH, resulting in inflammatory cascade reactions and aggravating brain injury. Furthermore, previous studies have reported that NLRP3 inflammasome is mainly present in microglia, so we speculate that its activation may be strongly associated with microglial polarization. Many scholars have investigated the role of brain injury caused by NLRP3 inflammasome after ICH, but the precise operating mechanisms remain uncertain. This review summarized the activation mechanism of NLRP3 inflammasome after ICH and the possible mechanism of NLRP3 inflammasome promoting neuroinflammation and aggravating nerve injury and discussed the relevant potential therapeutic targets.


Similar content being viewed by others
References
Katsuki H, Hijioka M (2017) Intracerebral hemorrhage as an axonal tract injury disorder with inflammatory reactions. Biol Pharm Bull 40(5):564–568. https://doi.org/10.1248/bpb.b16-01013
Qureshi AI, Mendelow AD, Hanley DF (2009) Intracerebral haemorrhage. Lancet 373(9675):1632–1644. https://doi.org/10.1016/S0140-6736(09)60371-8 Elsevier
Keep RF, Hua Y, Xi G (2012) Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol 11(8):720–731. https://doi.org/10.1016/s1474-4422(12)70104-7
Chen S, Yang Q, Chen G, Zhang JH (2015) An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res 6(1):4–8. https://doi.org/10.1007/s12975-014-0384-4
Zhou Y, Wang Y, Wang J, Anne Stetler R, Yang Q-W (2014) Inflammation in intracerebral hemorrhage: from mechanisms to clinical translation. Prog Neurobiol 115:25–44. https://doi.org/10.1016/j.pneurobio.2013.11.003
Hu X, Tao C, Gan Q, Zheng J, Li H, You C (2016, 2016) Oxidative stress in intracerebral hemorrhage: sources, mechanisms, and therapeutic targets. Oxidative Med Cell Longev:3215391. https://doi.org/10.1155/2016/3215391 Hindawi Publishing Corporation
Yin J, Lü TM, Qiu G, Huang RY, Fang M, Wang YY, Xiao D, Liu XJ (2013) Intracerebral hematoma extends via perivascular spaces and perineurium. Tohoku J Exp Med 230(3):133–139. https://doi.org/10.1620/tjem.230.133
Hemphill JCR, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN et al (2015) Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 46(7):2032–2060. https://doi.org/10.1161/str.0000000000000069
Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM, STICH II (2013) Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet (London, England) 382(9890):397–408. https://doi.org/10.1016/S0140-6736(13)60986-1 Lancet Publishing Group
Gonzales NR (2013) Ongoing clinical trials in intracerebral hemorrhage. Stroke 44(6 Suppl 1):S70–S73. https://doi.org/10.1161/strokeaha.111.000563
Dong R, Li F, Xu Y, Chen P, Maegele M, Yang H, Chen W (2018) Safety and efficacy of applying sufficient analgesia combined with a minimal sedation program as an early antihypertensive treatment for spontaneous intracerebral hemorrhage: a randomized controlled trial. Trials 19(1):607. https://doi.org/10.1186/s13063-018-2943-6
Zhang Y, Shan AJ, Peng YP, Lei P, Xu J, Zhong X, Du B (2019) The intra-neuroendoscopic technique (INET): a modified minimally invasive technique for evacuation of brain parenchyma hematomas. World J Emerg Surg 14:21. https://doi.org/10.1186/s13017-019-0239-0
Xi G, Keep RF, Hoff JT (2006) Mechanisms of brain injury after intracerebral haemorrhage. Lancet Neurol 5(1):53–63. https://doi.org/10.1016/s1474-4422(05)70283-0
Li J, Yan F, Chen G (2014) Reactive oxygen species and NLRP3 inflammasome activation. Ann Neurol 75(6):972. https://doi.org/10.1002/ana.24173
Fu Z, Chen Y, Qin F, Yang S, Deng X, Ding R, Feng L, Li W et al (2014) Increased activity of Rho kinase contributes to hemoglobin-induced early disruption of the blood-brain barrier in vivo after the occurrence of intracerebral hemorrhage. Int J Clin Exp Pathol 7(11):7844–7853
Caner B, Hou J, Altay O, Fujii M, Zhang JH (2012) Transition of research focus from vasospasm to early brain injury after subarachnoid hemorrhage. J Neurochem 123(Suppl 2):12–21. https://doi.org/10.1111/j.1471-4159.2012.07939.x
Egashira Y, Hua Y, Keep RF, Xi G (2015) Intercellular cross-talk in intracerebral hemorrhage. Brain Res 1623:97–109. https://doi.org/10.1016/j.brainres.2015.04.003
Tao C, Hu X, Li H, You C (2017) White matter injury after intracerebral hemorrhage: pathophysiology and therapeutic strategies. Front Hum Neurosci 11:422. https://doi.org/10.3389/fnhum.2017.00422
Cheng S, Gao W, Xu X, Fan H, Wu Y, Li F, Zhang J, Zhu X et al (2016) Methylprednisolone sodium succinate reduces BBB disruption and inflammation in a model mouse of intracranial haemorrhage. Brain Res Bull 127:226–233. https://doi.org/10.1016/j.brainresbull.2016.10.007
Askenase MH, Sansing LH (2016) Stages of the inflammatory response in pathology and tissue repair after intracerebral hemorrhage. Semin Neurol 36(3):288–297. https://doi.org/10.1055/s-0036-1582132
Ren H, Kong Y, Liu Z, Zang D, Yang X, Wood K, Li M, Liu Q (2018) Selective NLRP3 (pyrin domain–containing protein 3) inflammasome inhibitor reduces brain injury after intracerebral hemorrhage. Stroke 49(1):184–192. https://doi.org/10.1161/strokeaha.117.018904
Yang S-J, Shao G-F, Chen J-L, Gong J (2018) The NLRP3 inflammasome: an important driver of neuroinflammation in hemorrhagic stroke. Cell Mol Neurobiol 38(3):595–603. https://doi.org/10.1007/s10571-017-0526-9
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19(10):610–621. https://doi.org/10.1038/s41583-018-0055-7
Luo Y, Reis C, Chen S (2019) NLRP3 inflammasome in the pathophysiology of hemorrhagic stroke: a review. Curr Neuropharmacol 17(7):582–589. https://doi.org/10.2174/1570159X17666181227170053 Bentham Science Publishers
Cheng Y, Wei Y, Yang W, Song Y, Shang H, Cai Y, Wu Z, Zhao W (2017) Cordycepin confers neuroprotection in mice models of intracerebral hemorrhage via suppressing NLRP3 inflammasome activation. Metab Brain Dis 32(4):1133–1145. https://doi.org/10.1007/s11011-017-0003-7
Shao B-Z, Cao Q, Liu C (2018) Targeting NLRP3 inflammasome in the treatment of CNS diseases. Front Mol Neurosci 11:320. https://doi.org/10.3389/fnmol.2018.00320 Frontiers Media S.A
Zeng J, Chen Y, Ding R, Feng L, Fu Z, Yang S, Deng X, Xie Z et al (2017) Isoliquiritigenin alleviates early brain injury after experimental intracerebral hemorrhage via suppressing ROS- and/or NF-κB-mediated NLRP3 inflammasome activation by promoting Nrf2 antioxidant pathway. J Neuroinflammation 14(1):119. https://doi.org/10.1186/s12974-017-0895-5 BioMed Central
Wang J (2010) Preclinical and clinical research on inflammation after intracerebral hemorrhage. Prog Neurobiol 92(4):463–477. https://doi.org/10.1016/j.pneurobio.2010.08.001
Wang Y, Lin J, Chen QZ, Zhu N, Jiang DQ, Li MX, Wang Y (2015) Overexpression of mitochondrial Hsp75 protects neural stem cells against microglia-derived soluble factor-induced neurotoxicity by regulating mitochondrial permeability transition pore opening in vitro. Int J Mol Med 36(6):1487–1496. https://doi.org/10.3892/ijmm.2015.2380
Yue X, Qiao D, Wang A, Tan X, Li Y, Liu C, Wang H (2012) CD200 attenuates methamphetamine-induced microglial activation and dopamine depletion. J Huazhong Univ Sci Technol Med Sci 32(3):415–421. https://doi.org/10.1007/s11596-012-0072-0
Zhu N, Lin J, Wang K, Wei M, Chen Q, Wang Y (2015) Huperzine A protects neural stem cells against Aβ-induced apoptosis in a neural stem cells and microglia co-culture system. Int J Clin Exp Pathol 8(6):6425–6433
Brown GC, Vilalta A (2015) How microglia kill neurons. Brain Res 1628(Pt B):288–297. https://doi.org/10.1016/j.brainres.2015.08.031
Iadecola C, Anrather J (2011) The immunology of stroke: from mechanisms to translation. Nat Med 17(7):796–808. https://doi.org/10.1038/nm.2399
Xiong X-Y, Liu L, Yang Q-W (2016) Functions and mechanisms of microglia/macrophages in neuroinflammation and neurogenesis after stroke. Prog Neurobiol 142:23–44. https://doi.org/10.1016/j.pneurobio.2016.05.001
Fumagalli S, Perego C, Pischiutta F, Zanier ER, De Simoni M-G (2015) The ischemic environment drives microglia and macrophage function. Front Neurol 6:81. https://doi.org/10.3389/fneur.2015.00081 Frontiers Media S.A
Bouhlel MA, Derudas B, Rigamonti E, Dièvart R, Brozek J, Haulon S, Zawadzki C, Jude B et al (2007) PPARgamma activation primes human monocytes into alternative M2 macrophages with anti-inflammatory properties. Cell Metab 6(2):137–143. https://doi.org/10.1016/j.cmet.2007.06.010
Tentillier N, Etzerodt A, Olesen MN, Rizalar FS, Jacobsen J, Bender D, Moestrup SK, Romero-Ramos M (2016) Anti-inflammatory modulation of microglia via CD163-targeted glucocorticoids protects dopaminergic neurons in the 6-OHDA Parkinson’s disease model. J Neurosci 36(36):9375–9390. https://doi.org/10.1523/JNEUROSCI.1636-16.2016 Society for Neuroscience
Hu X, Li P, Guo Y, Wang H, Leak RK, Chen S, Gao Y, Chen J (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43(11):3063–3070. https://doi.org/10.1161/strokeaha.112.659656
Wang G, Zhang J, Hu X, Zhang L, Mao L, Jiang X, Liou AK-F, Leak RK et al (2013) Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. Cereb Blood Flow Metab 33(12):1864–1874. https://doi.org/10.1038/jcbfm.2013.146 Nature Publishing Group
Yang Y, Liu H, Zhang H, Ye Q, Wang J, Yang B, Mao L, Zhu W et al (2017) ST2/IL-33-dependent microglial response limits acute ischemic brain injury. J Neurosci 37(18):4692–4704. https://doi.org/10.1523/JNEUROSCI.3233-16.2017 Society for Neuroscience
Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R (2016) IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia 64(12):2079–2092. https://doi.org/10.1002/glia.23041
Zhang Z, Zhang Z, Lu H, Yang Q, Wu H, Wang J (2017) Microglial polarization and inflammatory mediators after intracerebral hemorrhage. Mol Neurobiol 54(3):1874–1886. https://doi.org/10.1007/s12035-016-9785-6
Shi H, Zheng K, Su Z, Su H, Zhong M, He X, Zhou C, Chen H et al (2016) Sinomenine enhances microglia M2 polarization and attenuates inflammatory injury in intracerebral hemorrhage. J Neuroimmunol 299:28–34. https://doi.org/10.1016/j.jneuroim.2016.08.010
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 10(3):241–247. https://doi.org/10.1038/ni.1703
Heneka MT, Kummer MP, Latz E (2014) Innate immune activation in neurodegenerative disease. Nat Rev Immunol 14(7):463–477. https://doi.org/10.1038/nri3705
Feng L, Chen Y, Ding R, Fu Z, Yang S, Deng X, Zeng J (2015) P2X7R blockade prevents NLRP3 inflammasome activation and brain injury in a rat model of intracerebral hemorrhage: involvement of peroxynitrite. J Neuroinflammation 12:190. https://doi.org/10.1186/s12974-015-0409-2
Ma Q, Chen S, Hu Q, Feng H, Zhang JH, Tang J (2014) NLRP3 inflammasome contributes to inflammation after intracerebral hemorrhage. Ann Neurol 75(2):209–219. https://doi.org/10.1002/ana.24070
Zaki MH, Lamkanfi M, Kanneganti TD (2011) The Nlrp3 inflammasome: contributions to intestinal homeostasis. Trends Immunol 32(4):171–179. https://doi.org/10.1016/j.it.2011.02.002 Copyright © 2011 Elsevier Ltd. All rights reserved
Bryant C, Fitzgerald KA (2009) Molecular mechanisms involved in inflammasome activation. Trends Cell Biol 19(9):455–464. https://doi.org/10.1016/j.tcb.2009.06.002
Mariathasan S, Monack DM (2007) Inflammasome adaptors and sensors: intracellular regulators of infection and inflammation. Nat Rev Immunol 7(1):31–40. https://doi.org/10.1038/nri1997
Lechtenberg BC, Mace PD, Riedl SJ (2014) Structural mechanisms in NLR inflammasome signaling. Curr Opin Struct Biol 29:17–25. https://doi.org/10.1016/j.sbi.2014.08.011
Lu A, Magupalli VG, Ruan J, Yin Q, Atianand MK, Vos MR, Schröder GF, Fitzgerald KA et al (2014) Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 156(6):1193–1206. https://doi.org/10.1016/j.cell.2014.02.008
Proell M, Gerlic M, Mace PD, Reed JC, Riedl SJ (2013) The CARD plays a critical role in ASC foci formation and inflammasome signalling. Biochem J 449(3):613–621. https://doi.org/10.1042/bj20121198
Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM (2013) Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. J Clin Invest 123(11):4695–4705. https://doi.org/10.1172/jci71543
Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, Hoffman HM, Feldstein AE (2014) NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 59(3):898–910. https://doi.org/10.1002/hep.26592
Segovia J, Sabbah A, Mgbemena V, Tsai SY, Chang TH, Berton MT, Morris IR, Allen IC et al (2012) TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PLoS One 7(1):e29695. https://doi.org/10.1371/journal.pone.0029695
Lamkanfi M (2011) Emerging inflammasome effector mechanisms. Nat Rev Immunol 11(3):213–220. https://doi.org/10.1038/nri2936
Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol 9(8):847–856. https://doi.org/10.1038/ni.1631
Muñoz-Planillo R, Kuffa P, Martínez-Colón G, Smith BL, Rajendiran TM, Núñez G (2013) K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity 38(6):1142–1153. https://doi.org/10.1016/j.immuni.2013.05.016 Copyright © 2013 Elsevier Inc. All rights reserved
Zhou R, Yazdi AS, Menu P, Tschopp J (2011) A role for mitochondria in NLRP3 inflammasome activation. Nature 469(7329):221–225. https://doi.org/10.1038/nature09663
Iyer SS, He Q, Janczy JR, Elliott EI, Zhong Z, Olivier AK, Sadler JJ, Knepper-Adrian V et al (2013) Mitochondrial cardiolipin is required for Nlrp3 inflammasome activation. Immunity 39(2):311–323. https://doi.org/10.1016/j.immuni.2013.08.001 Copyright © 2013 Elsevier Inc. All rights reserved
Dutra FF, Alves LS, Rodrigues D, Fernandez PL, de Oliveira RB, Golenbock DT, Zamboni DS, Bozza MT (2014) Hemolysis-induced lethality involves inflammasome activation by heme. Proc Natl Acad Sci U S A 111(39):E4110–E4118. https://doi.org/10.1073/pnas.1405023111
Yao ST, Cao F, Chen JL, Chen W, Fan RM, Li G, Zeng YC, Jiao S et al (2017) NLRP3 is required for complement-mediated caspase-1 and IL-1beta activation in ICH. J Mol Neurosci 61(3):385–395. https://doi.org/10.1007/s12031-016-0874-9
Yang Z, Zhong L, Xian R, Yuan B (2015) MicroRNA-223 regulates inflammation and brain injury via feedback to NLRP3 inflammasome after intracerebral hemorrhage. Mol Immunol 65(2):267–276. https://doi.org/10.1016/j.molimm.2014.12.018 Copyright © 2015. Published by Elsevier Ltd
Di Virgilio F (2007) Liaisons dangereuses: P2X(7) and the inflammasome. Trends Pharmacol Sci 28(9):465–472. https://doi.org/10.1016/j.tips.2007.07.002
Jiang T, Hoekstra J, Heng X, Kang W, Ding J, Liu J, Chen S, Zhang J (2015) P2X7 receptor is critical in α-synuclein--mediated microglial NADPH oxidase activation. Neurobiol Aging 36(7):2304–2318. https://doi.org/10.1016/j.neurobiolaging.2015.03.015 Copyright © 2015 Elsevier Inc. All rights reserved
Zhao H, Pan P, Yang Y, Ge H, Chen W, Qu J, Shi J, Cui G et al (2017) Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J Neuroinflammation 14(1):163. https://doi.org/10.1186/s12974-017-0940-4
Ye X, Zuo D, Yu L, Zhang L, Tang J, Cui C, Bao L, Zan K et al (2017) ROS/TXNIP pathway contributes to thrombin induced NLRP3 inflammasome activation and cell apoptosis in microglia. Biochem Biophys Res Commun 485(2):499–505. https://doi.org/10.1016/j.bbrc.2017.02.019 Copyright © 2017 Elsevier Inc. All rights reserved
Ismael S, Nasoohi S, Yoo A, Ahmed HA, Ishrat T (2020) Tissue plasminogen activator promotes TXNIP-NLRP3 inflammasome activation after hyperglycemic stroke in mice. Mol Neurobiol. https://doi.org/10.1007/s12035-020-01893-7
Hu L, Zhang H, Wang B, Ao Q, He Z (2020) MicroRNA-152 attenuates neuroinflammation in intracerebral hemorrhage by inhibiting thioredoxin interacting protein (TXNIP)-mediated NLRP3 inflammasome activation. Int Immunopharmacol 80:106141. https://doi.org/10.1016/j.intimp.2019.106141 Copyright © 2020 Elsevier B.V. All rights reserved
Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J (2010) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol 11(2):136–140. https://doi.org/10.1038/ni.1831
Calkins MJ, Johnson DA, Townsend JA, Vargas MR, Dowell JA, Williamson TP, Kraft AD, Lee JM et al (2009) The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid Redox Signal 11(3):497–508. https://doi.org/10.1089/ars.2008.2242
Denzer I, Münch G, Friedland K (2016) Modulation of mitochondrial dysfunction in neurodegenerative diseases via activation of nuclear factor erythroid-2-related factor 2 by food-derived compounds. Pharmacol Res 103:80–94. https://doi.org/10.1016/j.phrs.2015.11.019 Copyright © 2015 Elsevier Ltd. All rights reserved
Shang H, Yang D, Zhang W, Li T, Ren X, Wang X, Zhao W (2013) Time course of Keap1-Nrf2 pathway expression after experimental intracerebral haemorrhage: correlation with brain oedema and neurological deficit. Free Radic Res 47(5):368–375. https://doi.org/10.3109/10715762.2013.778403
Iniaghe LO, Krafft PR, Klebe DW, Omogbai E, Zhang JH, Tang J (2015) Dimethyl fumarate confers neuroprotection by casein kinase 2 phosphorylation of Nrf2 in murine intracerebral hemorrhage. Neurobiol Dis 82:349–358. https://doi.org/10.1016/j.nbd.2015.07.001 Copyright © 2015 Elsevier Inc. All rights reserved
Wang J, Fields J, Zhao C, Langer J, Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S et al (2007) Role of Nrf2 in protection against intracerebral hemorrhage injury in mice. Free Radic Biol Med 43(3):408–414. https://doi.org/10.1016/j.freeradbiomed.2007.04.020
Zhao X, Sun G, Zhang J, Strong R, Dash PK, Kan YW, Grotta JC, Aronowski J (2007) Transcription factor Nrf2 protects the brain from damage produced by intracerebral hemorrhage. Stroke 38(12):3280–3286. https://doi.org/10.1161/strokeaha.107.486506
Zhao X, Sun G, Zhang J, Ting SM, Gonzales N, Aronowski J (2015) Dimethyl fumarate protects brain from damage produced by intracerebral hemorrhage by mechanism involving Nrf2. Stroke 46(7):1923–1928. https://doi.org/10.1161/STROKEAHA.115.009398 © 2015 American Heart Association, Inc
Zhao X, Sun G, Ting SM, Song S, Zhang J, Edwards NJ, Aronowski J (2015) Cleaning up after ICH: the role of Nrf2 in modulating microglia function and hematoma clearance. J Neurochem 133(1):144–152. https://doi.org/10.1111/jnc.12974 © 2014 International Society for Neurochemistry
Yuan R, Fan H, Cheng S, Gao W, Xu X, Lv S, Ye M, Wu M et al (2017) Silymarin prevents NLRP3 inflammasome activation and protects against intracerebral hemorrhage. Biomed Pharmacother 93:308–315. https://doi.org/10.1016/j.biopha.2017.06.018 Copyright © 2017 Elsevier Masson SAS. All rights reserved
Cheng Y, Chen B, Xie W, Chen Z, Yang G, Cai Y, Shang H, Zhao W (2020) Ghrelin attenuates secondary brain injury following intracerebral hemorrhage by inhibiting NLRP3 inflammasome activation and promoting Nrf2/ARE signaling pathway in mice. Int Immunopharmacol 79:106180. https://doi.org/10.1016/j.intimp.2019.106180 Copyright © 2020 Elsevier B.V. All rights reserved
Naito Y, Takagi T, Higashimura Y (2014) Heme oxygenase-1 and anti-inflammatory M2 macrophages. Arch Biochem Biophys 564:83–88. https://doi.org/10.1016/j.abb.2014.09.005
Asgari E, Le Friec G, Yamamoto H, Perucha E, Sacks SS, Köhl J, Cook HT, Kemper C (2013) C3a modulates IL-1β secretion in human monocytes by regulating ATP efflux and subsequent NLRP3 inflammasome activation. Blood 122(20):3473–3481. https://doi.org/10.1182/blood-2013-05-502229
Hollmann TJ, Haviland DL, Kildsgaard J, Watts K, Wetsel RA (1998) Cloning, expression, sequence determination, and chromosome localization of the mouse complement C3a anaphylatoxin receptor gene. Mol Immunol 35(3):137–148. https://doi.org/10.1016/s0161-5890(98)00021-2
Nataf S, Stahel PF, Davoust N, Barnum SR (1999) Complement anaphylatoxin receptors on neurons: new tricks for old receptors? Trends Neurosci 22(9):397–402. https://doi.org/10.1016/s0166-2236(98)01390-3
Ames RS, Li Y, Sarau HM, Nuthulaganti P, Foley JJ, Ellis C, Zeng Z, Su K et al (1996) Molecular cloning and characterization of the human anaphylatoxin C3a receptor. J Biol Chem 271(34):20231–20234. https://doi.org/10.1074/jbc.271.34.20231
Gasque P, Singhrao SK, Neal JW, Wang P, Sayah S, Fontaine M, Morgan BP (1998) The receptor for complement anaphylatoxin C3a is expressed by myeloid cells and nonmyeloid cells in inflamed human central nervous system: analysis in multiple sclerosis and bacterial meningitis. J Immunol 160(7):3543–3554
Rynkowski MA, Kim GH, Garrett MC, Zacharia BE, Otten ML, Sosunov SA, Komotar RJ, Hassid BG et al (2009) C3a receptor antagonist attenuates brain injury after intracerebral hemorrhage. J Cereb Blood Flow Metab 29(1):98–107. https://doi.org/10.1038/jcbfm.2008.95
Garrett MC, Otten ML, Starke RM, Komotar RJ, Magotti P, Lambris JD, Rynkowski MA, Connolly ES (2009) Synergistic neuroprotective effects of C3a and C5a receptor blockade following intracerebral hemorrhage. Brain Res 1298:171–177. https://doi.org/10.1016/j.brainres.2009.04.047
Haggadone MD, Grailer JJ, Fattahi F, Zetoune FS, Ward PA (2016) Bidirectional crosstalk between C5a receptors and the NLRP3 inflammasome in macrophages and monocytes. Mediat Inflamm 2016:1340156. https://doi.org/10.1155/2016/1340156
Arbore G, Kemper C (2016) A novel “complement-metabolism-inflammasome axis” as a key regulator of immune cell effector function. Eur J Immunol 46(7):1563–1573. https://doi.org/10.1002/eji.201546131
Ju H, Li X, Li H, Wang X, Wang H, Li Y, Dou C, Zhao G (2013) Mediation of multiple pathways regulating cell proliferation, migration, and apoptosis in the human malignant glioma cell line U87MG via unphosphorylated STAT1: laboratory investigation. J Neurosurg 118(6):1239–1247. https://doi.org/10.3171/2013.3.Jns122051
Liang Z, Wu G, Fan C, Xu J, Jiang S, Yan X, Di S, Ma Z et al (2016) The emerging role of signal transducer and activator of transcription 3 in cerebral ischemic and hemorrhagic stroke. Prog Neurobiol 137:1–16. https://doi.org/10.1016/j.pneurobio.2015.11.001
Arimoto K-I, Löchte S, Stoner SA, Burkart C, Zhang Y, Miyauchi S, Wilmes S, Fan J-B et al (2017) STAT2 is an essential adaptor in USP18-mediated suppression of type I interferon signaling. Nat Struct Mol Biol 24(3):279–289. https://doi.org/10.1038/nsmb.3378
Jeon S-B, Yoon HJ, Chang CY, Koh HS, Jeon S-H, Park EJ (2010) Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol (Baltimore, Md : 1950) 185(11):7037–7046. https://doi.org/10.4049/jimmunol.1000154
Lan X, Han X, Li Q, Yang QW, Wang J (2017) Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol 13(7):420–433. https://doi.org/10.1038/nrneurol.2017.69
Lawrence T, Natoli G (2011) Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol 11(11):750–761. https://doi.org/10.1038/nri3088
Leopold Wager CM, Hole CR, Wozniak KL, Olszewski MA, Mueller M, Wormley FLJ (2015) STAT1 signaling within macrophages is required for antifungal activity against Cryptococcus neoformans. Infect Immun 83(12):4513–4527. https://doi.org/10.1128/IAI.00935-15 American Society for Microbiology
Nguyen H, Ramana CV, Bayes J, Stark GR (2001) Roles of phosphatidylinositol 3-kinase in interferon-gamma-dependent phosphorylation of STAT1 on serine 727 and activation of gene expression. J Biol Chem 276(36):33361–33368. https://doi.org/10.1074/jbc.M105070200
Qin H, Yeh W-I, De Sarno P, Holdbrooks AT, Liu Y, Muldowney MT, Reynolds SL, Yanagisawa LL et al (2012) Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc Natl Acad Sci U S A 109(13):5004–5009. https://doi.org/10.1073/pnas.1117218109 National Academy of Sciences
Wang Y, Han Z, Fan Y, Zhang J, Chen K, Gao L, Zeng H, Cao J et al (2017) MicroRNA-9 inhibits NLRP3 inflammasome activation in human atherosclerosis inflammation cell models through the JAK1/STAT signaling pathway. Cell Physiol Biochem 41(4):1555–1571. https://doi.org/10.1159/000470822
Guarda G, Braun M, Staehli F, Tardivel A, Mattmann C, Förster I, Farlik M, Decker T et al (2011) Type I interferon inhibits interleukin-1 production and inflammasome activation. Immunity 34(2):213–223. https://doi.org/10.1016/j.immuni.2011.02.006 Copyright © 2011 Elsevier Inc. All rights reserved
Wang T, Nowrangi D, Yu L, Lu T, Tang J, Han B, Ding Y, Fu F et al (2018) Activation of dopamine D1 receptor decreased NLRP3-mediated inflammation in intracerebral hemorrhage mice. J Neuroinflammation 15(1):2. https://doi.org/10.1186/s12974-017-1039-7 BioMed Central
Li R, Liu W, Yin J, Chen Y, Guo S, Fan H, Li X, Zhang X et al (2018) TSG-6 attenuates inflammation-induced brain injury via modulation of microglial polarization in SAH rats through the SOCS3/STAT3 pathway. J Neuroinflammation 15(1):231. https://doi.org/10.1186/s12974-018-1279-1 BioMed Central
Tsai MC, Chen WJ, Tsai MS, Ching CH, Chuang JI (2011) Melatonin attenuates brain contusion-induced oxidative insult, inactivation of signal transducers and activators of transcription 1, and upregulation of suppressor of cytokine signaling-3 in rats. J Pineal Res 51(2):233–245. https://doi.org/10.1111/j.1600-079X.2011.00885.x
Kim CK, Ryu W-S, Choi I-Y, Kim Y-J, Rim D, Kim BJ, Jang H, Yoon B-W et al (2013) Detrimental effects of leptin on intracerebral hemorrhage via the STAT3 signal pathway. J Cereb Blood Flow Metab 33(6):944–953. https://doi.org/10.1038/jcbfm.2013.35 Nature Publishing Group
Hasegawa M, Imamura R, Motani K, Nishiuchi T, Matsumoto N, Kinoshita T, Suda T (2009) Mechanism and repertoire of ASC-mediated gene expression. J Immunol (Baltimore, Md : 1950) 182(12):7655–7662. https://doi.org/10.4049/jimmunol.0800448
Uhlemann R, Gertz K, Boehmerle W, Schwarz T, Nolte C, Freyer D, Kettenmann H, Endres M et al (2016) Actin dynamics shape microglia effector functions. Brain Struct Funct 221(5):2717–2734. https://doi.org/10.1007/s00429-015-1067-y
Ferreira R, Lively S, Schlichter LC (2014) IL-4 type 1 receptor signaling up-regulates KCNN4 expression, and increases the KCa3.1 current and its contribution to migration of alternative-activated microglia. Front Cell Neurosci 8:183. https://doi.org/10.3389/fncel.2014.00183 Frontiers Media S.A
Freilich RW, Woodbury ME, Ikezu T (2013) Integrated expression profiles of mRNA and miRNA in polarized primary murine microglia. PLoS One 8(11):e79416. https://doi.org/10.1371/journal.pone.0079416 Public Library of Science
Fang R, Uchiyama R, Sakai S, Hara H, Tsutsui H, Suda T, Mitsuyama M, Kawamura I et al (2019) ASC and NLRP3 maintain innate immune homeostasis in the airway through an inflammasome-independent mechanism. Mucosal Immunol 12(5):1092–1103. https://doi.org/10.1038/s41385-019-0181-1
Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS et al (2015) A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21(3):248–255. https://doi.org/10.1038/nm.3806
Lamkanfi M, Mueller JL, Vitari AC, Misaghi S, Fedorova A, Deshayes K, Lee WP, Hoffman HM et al (2009) Glyburide inhibits the cryopyrin/Nalp3 inflammasome. J Cell Biol 187(1):61–70. https://doi.org/10.1083/jcb.200903124
Orhan N, Ugur YC, Ekizoglu O, Ahishali B, Kucuk M, Arican N, Elmas I, Gürses C et al (2016) Effects of beta-hydroxybutyrate on brain vascular permeability in rats with traumatic brain injury. Brain Res 1631:113–126. https://doi.org/10.1016/j.brainres.2015.11.038 Copyright © 2015 Elsevier B.V. All rights reserved
Dempsey C, Rubio AA, Bryson KJ, Finucane O, Larkin C, Mills EL, Robertson A, Cooper MA et al (2017) Inhibiting the NLRP3 inflammasome with MCC950 promotes non-phlogistic clearance of amyloid-β and cognitive function in APP/PS1 mice. Brain Behav Immun 61:306–316. https://doi.org/10.1016/j.bbi.2016.12.014 Copyright © 2016 Elsevier Inc. All rights reserved
Khan N, Kuo A, Brockman DA, Cooper MA, Smith MT (2018) Pharmacological inhibition of the NLRP3 inflammasome as a potential target for multiple sclerosis induced central neuropathic pain. Inflammopharmacology 26(1):77–86. https://doi.org/10.1007/s10787-017-0401-9
Xu X, Yin D, Ren H, Gao W, Li F, Sun D, Wu Y, Zhou S et al (2018) Selective NLRP3 inflammasome inhibitor reduces neuroinflammation and improves long-term neurological outcomes in a murine model of traumatic brain injury. Neurobiol Dis 117:15–27. https://doi.org/10.1016/j.nbd.2018.05.016 Copyright © 2018. Published by Elsevier Inc
Luo Y, Lu J, Ruan W, Guo X, Chen S (2019) MCC950 attenuated early brain injury by suppressing NLRP3 inflammasome after experimental SAH in rats. Brain Res Bull 146:320–326. https://doi.org/10.1016/j.brainresbull.2019.01.027 Copyright © 2019 Elsevier Inc. All rights reserved
Hill JR, Coll RC, Sue N, Reid JC, Dou J, Holley CL, Pelingon R, Dickinson JB et al (2017) Sulfonylureas as concomitant insulin secretagogues and NLRP3 inflammasome inhibitors. ChemMedChem 12(17):1449–1457. https://doi.org/10.1002/cmdc.201700270 © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim
Juliana C, Fernandes-Alnemri T, Wu J, Datta P, Solorzano L, Yu JW, Meng R, Quong AA et al (2010) Anti-inflammatory compounds parthenolide and Bay 11-7082 are direct inhibitors of the inflammasome. J Biol Chem 285(13):9792–9802. https://doi.org/10.1074/jbc.M109.082305
Dong L, Qiao H, Zhang X, Zhang X, Wang C, Wang L, Cui L, Zhao J et al (2013) Parthenolide is neuroprotective in rat experimental stroke model: downregulating NF-κB, phospho-p38MAPK, and caspase-1 and ameliorating BBB permeability. Mediat Inflamm 2013:370804. https://doi.org/10.1155/2013/370804
Wang JA, Tong ML, Zhao B, Zhu G, Xi DH, Yang JP (2020) Parthenolide ameliorates intracerebral hemorrhage-induced brain injury in rats. Phytother Res 34(1):153–160. https://doi.org/10.1002/ptr.6510 © 2019 John Wiley & Sons, Ltd
Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D‘Agostino D, Planavsky N et al (2015) The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med 21(3):263–269. https://doi.org/10.1038/nm.3804
Bae HR, Kim DH, Park MH, Lee B, Kim MJ, Lee EK, Chung KW, Kim SM et al (2016) β-Hydroxybutyrate suppresses inflammasome formation by ameliorating endoplasmic reticulum stress via AMPK activation. Oncotarget 7(41):66444–66454. https://doi.org/10.18632/oncotarget.12119
Xie G, Tian W, Wei T, Liu F (2015) The neuroprotective effects of β-hydroxybutyrate on Aβ-injected rat hippocampus in vivo and in Aβ-treated PC-12 cells in vitro. Free Radic Res 49(2):139–150. https://doi.org/10.3109/10715762.2014.987274
Guo C, Fulp JW, Jiang Y, Li X, Chojnacki JE, Wu J, Wang XY, Zhang S (2017) Development and characterization of a hydroxyl-sulfonamide analogue, 5-chloro-N-[2-(4-hydroxysulfamoyl-phenyl)-ethyl]-2-methoxy-benzamide, as a novel NLRP3 inflammasome inhibitor for potential treatment of multiple sclerosis. ACS Chem Neurosci 8(10):2194–2201. https://doi.org/10.1021/acschemneuro.7b00124
Flores J, Noël A, Foveau B, Lynham J, Lecrux C, LeBlanc AC (2018) Caspase-1 inhibition alleviates cognitive impairment and neuropathology in an Alzheimer’s disease mouse model. Nat Commun 9(1):3916. https://doi.org/10.1038/s41467-018-06449-x
MacKenzie SH, Schipper JL, Clark AC (2010) The potential for caspases in drug discovery. Curr Opin Drug Discov Dev 13(5):568–576
Duncan JA, Bergstralh DT, Wang Y, Willingham SB, Ye Z, Zimmermann AG, Ting JP (2007) Cryopyrin/NALP3 binds ATP/dATP, is an ATPase, and requires ATP binding to mediate inflammatory signaling. Proc Natl Acad Sci U S A 104(19):8041–8046. https://doi.org/10.1073/pnas.0611496104
Pan Z, Shan Q, Gu P, Wang XM, Tai LW, Sun M, Luo X, Sun L et al (2018) miRNA-23a/CXCR4 regulates neuropathic pain via directly targeting TXNIP/NLRP3 inflammasome axis. J Neuroinflammation 15(1):29. https://doi.org/10.1186/s12974-018-1073-0
Chuang YT, Lin YC, Lin KH, Chou TF, Kuo WC, Yang KT, Wu PR, Chen RH et al (2011) Tumor suppressor death-associated protein kinase is required for full IL-1β production. Blood 117(3):960–970. https://doi.org/10.1182/blood-2010-08-303115
Cocco M, Garella D, Di Stilo A, Borretto E, Stevanato L, Giorgis M, Marini E, Fantozzi R et al (2014) Electrophilic warhead-based design of compounds preventing NLRP3 inflammasome-dependent pyroptosis. J Med Chem 57(24):10366–10382. https://doi.org/10.1021/jm501072b
He Y, Varadarajan S, Muñoz-Planillo R, Burberry A, Nakamura Y, Núñez G (2014) 3,4-methylenedioxy-β-nitrostyrene inhibits NLRP3 inflammasome activation by blocking assembly of the inflammasome. J Biol Chem 289(2):1142–1150. https://doi.org/10.1074/jbc.M113.515080
Irrera N, Pizzino G, Calò M, Pallio G, Mannino F, Famà F, Arcoraci V, Fodale V et al (2017) Lack of the Nlrp3 inflammasome improves mice recovery following traumatic brain injury. Front Pharmacol 8:459. https://doi.org/10.3389/fphar.2017.00459
Zheng B, Zhang S, Ying Y, Guo X, Li H, Xu L, Ruan X (2018) Administration of dexmedetomidine inhibited NLRP3 inflammasome and microglial cell activities in hippocampus of traumatic brain injury rats. Biosci Rep 38(5). https://doi.org/10.1042/BSR20180892 © 2018 The Author(s)
Mastrocola R, Penna C, Tullio F, Femminò S, Nigro D, Chiazza F, Serpe L, Collotta D et al (2016) Pharmacological inhibition of NLRP3 inflammasome attenuates myocardial ischemia/reperfusion injury by activation of RISK and mitochondrial pathways. Oxidative Med Cell Longev 2016:5271251. https://doi.org/10.1155/2016/5271251
Xiao M, Li L, Li C, Liu L, Yu Y, Ma L (2016) 3,4-Methylenedioxy-β-nitrostyrene ameliorates experimental burn wound progression by inhibiting the NLRP3 inflammasome activation. Plast Reconstr Surg 137(3):566e–575e. https://doi.org/10.1097/01.prs.0000479972.06934.83
Hsieh PW, Chang YT, Chuang WY, Shih HC, Chiang SZ, Wu CC (2010) The synthesis and biologic evaluation of anti-platelet and cytotoxic β-nitrostyrenes. Bioorg Med Chem 18(21):7621–7627. https://doi.org/10.1016/j.bmc.2010.08.039 Copyright © 2010 Elsevier Ltd. All rights reserved
Haque ME, Akther M, Jakaria M, Kim IS, Azam S, Choi DK (2020) Targeting the microglial NLRP3 inflammasome and its role in Parkinson’s disease. Mov Disord 35(1):20–33. https://doi.org/10.1002/mds.27874
Ma L, Wei L, Wu F, Hu Z, Liu Z, Yuan W (2013) Advances with microRNAs in Parkinson’s disease research. Drug Des Dev Ther 7:1103–1113. https://doi.org/10.2147/dddt.S48500
Yuan B, Shen H, Lin L, Su T, Zhong S, Yang Z (2015) Recombinant adenovirus encoding NLRP3 RNAi attenuate inflammation and brain injury after intracerebral hemorrhage. J Neuroimmunol 287:71–75. https://doi.org/10.1016/j.jneuroim.2015.08.002 Copyright © 2015 Elsevier B.V. All rights reserved
Qu X, Gao H, Tao L, Zhang Y, Zhai J, Song Y, Zhang S (2018) Autophagy inhibition-enhanced assembly of the NLRP3 inflammasome is associated with cisplatin-induced acute injury to the liver and kidneys in rats. J Biochem Mol Toxicol:e22208. https://doi.org/10.1002/jbt.22228 © 2018 Wiley Periodicals, Inc
Shao BZ, Wei W, Ke P, Xu ZQ, Zhou JX, Liu C (2014) Activating cannabinoid receptor 2 alleviates pathogenesis of experimental autoimmune encephalomyelitis via activation of autophagy and inhibiting NLRP3 inflammasome. CNS Neurosci Ther 20(12):1021–1028. https://doi.org/10.1111/cns.12349 © 2014 John Wiley & Sons Ltd
Chang YP, Ka SM, Hsu WH, Chen A, Chao LK, Lin CC, Hsieh CC, Chen MC et al (2015) Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J Cell Physiol 230(7):1567–1579. https://doi.org/10.1002/jcp.24903 © 2014 Wiley Periodicals, Inc
Zhang X, Wu Q, Zhang Q, Lu Y, Liu J, Li W, Lv S, Zhou M et al (2017) Resveratrol attenuates early brain injury after experimental subarachnoid hemorrhage via inhibition of NLRP3 inflammasome activation. Front Neurosci 11:611. https://doi.org/10.3389/fnins.2017.00611
Bonsack F, Alleyne CJ, Sukumari-Ramesh S (2017) Resveratrol attenuates neurodegeneration and improves neurological outcomes after intracerebral hemorrhage in mice. Front Cell Neurosci 11:228. https://doi.org/10.3389/fncel.2017.00228
Cai JC, Liu W, Lu F, Kong WB, Zhou XX, Miao P, Lei CX, Wang Y (2018) Resveratrol attenuates neurological deficit and neuroinflammation following intracerebral hemorrhage. Exp Ther Med 15(5):4131–4138. https://doi.org/10.3892/etm.2018.5938
Li L, Yun D, Zhang Y, Tao Y, Tan Q, Qiao F, Luo B, Liu Y et al (2018) A cannabinoid receptor 2 agonist reduces blood-brain barrier damage via induction of MKP-1 after intracerebral hemorrhage in rats. Brain Res 1697:113–123. https://doi.org/10.1016/j.brainres.2018.06.006 Copyright © 2018. Published by Elsevier B.V
Shao BZ, Xu ZQ, Han BZ, Su DF, Liu C (2015) NLRP3 inflammasome and its inhibitors: a review. Front Pharmacol 6:262. https://doi.org/10.3389/fphar.2015.00262
Lin L, Yihao T, Zhou F, Yin N, Qiang T, Haowen Z, Qianwei C, Jun T et al (2017) Inflammatory regulation by driving microglial M2 polarization: neuroprotective effects of cannabinoid receptor-2 activation in intracerebral hemorrhage. Front Immunol 8:112. https://doi.org/10.3389/fimmu.2017.00112
Alcocer-Gómez E, Casas-Barquero N, Williams MR, Romero-Guillena SL, Cañadas-Lozano D, Bullón P, Sánchez-Alcazar JA, Navarro-Pando JM et al (2017) Antidepressants induce autophagy dependent-NLRP3-inflammasome inhibition in Major depressive disorder. Pharmacol Res 121:114–121. https://doi.org/10.1016/j.phrs.2017.04.028 Copyright © 2017 Elsevier Ltd. All rights reserved
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A, Griep A, Axt D et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493(7434):674–678. https://doi.org/10.1038/nature11729
Zhou H, Feng L, Xu F, Sun Y, Ma Y, Zhang X, Liu H, Xu G et al (2017) Berberine inhibits palmitate-induced NLRP3 inflammasome activation by triggering autophagy in macrophages: a new mechanism linking berberine to insulin resistance improvement. Biomed Pharmacother 89:864–874. https://doi.org/10.1016/j.biopha.2017.03.003 Copyright © 2017. Published by Elsevier Masson SAS
Cui Y, Duan X, Li H, Dang B, Yin J, Wang Y, Gao A, Yu Z et al (2016) Hydrogen sulfide ameliorates early brain injury following subarachnoid hemorrhage in rats. Mol Neurobiol 53(6):3646–3657. https://doi.org/10.1007/s12035-015-9304-1
Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16(3):1066–1071. https://doi.org/10.1523/jneurosci.16-03-01066.1996
Lee M, McGeer EG, McGeer PL (2016) Sodium thiosulfate attenuates glial-mediated neuroinflammation in degenerative neurological diseases. J Neuroinflammation 13:32. https://doi.org/10.1186/s12974-016-0488-8
Liu H, Wang Y, Xiao Y, Hua Z, Cheng J, Jia J (2016) Hydrogen sulfide attenuates tissue plasminogen activator-induced cerebral hemorrhage following experimental stroke. Transl Stroke Res 7(3):209–219. https://doi.org/10.1007/s12975-016-0459-5
Ji J, Xiang P, Li T, Lan L, Xu X, Lu G, Ji H, Zhang Y et al (2017) NOSH-NBP, a novel nitric oxide and hydrogen sulfide-releasing hybrid, attenuates ischemic stroke-induced neuroinflammatory injury by modulating microglia polarization. Front Cell Neurosci 11:154. https://doi.org/10.3389/fncel.2017.00154
Castelblanco M, Lugrin J, Ehirchiou D, Nasi S, Ishii I, So A, Martinon F, Busso N (2018) Hydrogen sulfide inhibits NLRP3 inflammasome activation and reduces cytokine production both in vitro and in a mouse model of inflammation. J Biol Chem 293(7):2546–2557. https://doi.org/10.1074/jbc.M117.806869
Jia Q, Mehmood S, Liu X, Ma S, Yang R (2020) Hydrogen sulfide mitigates myocardial inflammation by inhibiting nucleotide-binding oligomerization domain-like receptor protein 3 inflammasome activation in diabetic rats. Exp Biol Med (Maywood) 245(3):221–230. https://doi.org/10.1177/1535370219899899
Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X, Zhang JH (2012) Hydrogen gas ameliorates oxidative stress in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med 40(4):1291–1296. https://doi.org/10.1097/CCM.0b013e31823da96d
Zhuang Z, Sun XJ, Zhang X, Liu HD, You WC, Ma CY, Zhu L, Zhou ML et al (2013) Nuclear factor-κB/Bcl-XL pathway is involved in the protective effect of hydrogen-rich saline on the brain following experimental subarachnoid hemorrhage in rabbits. J Neurosci Res 91(12):1599–1608. https://doi.org/10.1002/jnr.23281
Zhuang Z, Zhou ML, You WC, Zhu L, Ma CY, Sun XJ, Shi JX (2012) Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci 13:47. https://doi.org/10.1186/1471-2202-13-47
Zhuang K, Zuo YC, Sherchan P, Wang JK, Yan XX, Liu F (2019) Hydrogen inhalation attenuates oxidative stress related endothelial cells injury after subarachnoid hemorrhage in rats. Front Neurosci 13:1441. https://doi.org/10.3389/fnins.2019.01441
Manaenko A, Lekic T, Ma Q, Ostrowski RP, Zhang JH, Tang J (2011) Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir Suppl 111:179–183. https://doi.org/10.1007/978-3-7091-0693-8_30
Diaz-Hernandez JI, Gomez-Villafuertes R, León-Otegui M, Hontecillas-Prieto L, Del PA, Trejo JL, Lucas JJ, Garrido JJ et al (2012) In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer’s disease through GSK3β and secretases. Neurobiol Aging 33(8):1816–1828. https://doi.org/10.1016/j.neurobiolaging.2011.09.040 Copyright © 2012 Elsevier Inc. All rights reserved
Chen S, Ma Q, Krafft PR, Hu Q, Rolland W 2nd, Sherchan P, Zhang J, Tang J et al (2013) P2X7R/cryopyrin inflammasome axis inhibition reduces neuroinflammation after SAH. Neurobiol Dis 58:296–307. https://doi.org/10.1016/j.nbd.2013.06.011
Franke H, Günther A, Grosche J, Schmidt R, Rossner S, Reinhardt R, Faber-Zuschratter H, Schneider D et al (2004) P2X7 receptor expression after ischemia in the cerebral cortex of rats. J Neuropathol Exp Neurol 63(7):686–699. https://doi.org/10.1093/jnen/63.7.686
Meylan E, Tschopp J, Karin M (2006) Intracellular pattern recognition receptors in the host response. Nature 442(7098):39–44. https://doi.org/10.1038/nature04946
Inoue M, Williams KL, Oliver T, Vandenabeele P, Rajan JV, Miao EA, Shinohara ML (2012) Interferon-β therapy against EAE is effective only when development of the disease depends on the NLRP3 inflammasome. Sci Signal 5(225):ra38. https://doi.org/10.1126/scisignal.2002767
Malhotra S, Río J, Urcelay E, Nurtdinov R, Bustamante MF, Fernández O, Oliver B, Zettl U et al (2015) NLRP3 inflammasome is associated with the response to IFN-β in patients with multiple sclerosis. Brain 138(Pt 3):644–652. https://doi.org/10.1093/brain/awu388 © The Author (2015). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Shi JQ, Zhang CC, Sun XL, Cheng XX, Wang JB, Zhang YD, Xu J, Zou HQ (2013) Antimalarial drug artemisinin extenuates amyloidogenesis and neuroinflammation in APPswe/PS1dE9 transgenic mice via inhibition of nuclear factor-κB and NLRP3 inflammasome activation. CNS Neurosci Ther 19(4):262–268. https://doi.org/10.1111/cns.12066 © 2013 Blackwell Publishing Ltd
Silverman WR, de Rivero VJ, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G (2009) The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem 284(27):18143–18151. https://doi.org/10.1074/jbc.M109.004804
Jian Z, Ding S, Deng H, Wang J, Yi W, Wang L, Zhu S, Gu L et al (2016) Probenecid protects against oxygen-glucose deprivation injury in primary astrocytes by regulating inflammasome activity. Brain Res 1643:123–129. https://doi.org/10.1016/j.brainres.2016.05.002 Copyright © 2016 Elsevier B.V. All rights reserved
Mawhinney LJ, de Rivero Vaccari JP, Dale GA, Keane RW, Bramlett HM (2011) Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci 12:123. https://doi.org/10.1186/1471-2202-12-123
Daniels MJ, Rivers-Auty J, Schilling T, Spencer NG, Watremez W, Fasolino V, Booth SJ, White CS et al (2016) Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer’s disease in rodent models. Nat Commun 7:12504. https://doi.org/10.1038/ncomms12504
Acknowledgments
The support from National Natural Science Foundation of China is gratefully acknowledged.
Funding
This research was funded by the National Natural Science Foundation of China (81701243), the Natural Science Fund of Guangdong Province (2020A1515010038), the Pearl River S&T Nova Program of Guangzhou (201710010047), and the Presidential Foundation of Zhujiang Hospital of Southern Medical University (No. yzjj2018rc03).
Author information
Authors and Affiliations
Contributions
The work presented here was carried out in collaboration among all authors. H. S. and Q. W. conceived and designed the review; L. X., H. Z., and J. L. wrote the paper. All authors read, commented on, and approved this manuscript.
Corresponding authors
Ethics declarations
Conflicts of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, L., Zheng, H., Li, J. et al. Neuroinflammation Mediated by NLRP3 Inflammasome After Intracerebral Hemorrhage and Potential Therapeutic Targets. Mol Neurobiol 57, 5130–5149 (2020). https://doi.org/10.1007/s12035-020-02082-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-020-02082-2