Abstract
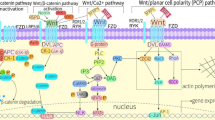
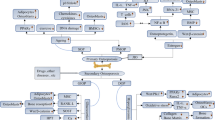
Osteoporosis (OP) is a metabolic bone disease linked to an elevated fracture risk, primarily stemming from disruptions in bone metabolism. Present clinical treatments for OP merely alleviate symptoms. Hence, there exists a pressing need to identify novel targets for the clinical treatment of OP. Research indicates that the Wnt signalling pathway is modulated by serum-secreted frizzled-related protein 5 (SFRP5), potentially serving as a pivotal regulator in bone metabolism disorders. Moreover, studies confirm elevated SFRP5 expression in OP, with SFRP5 overexpression leading to the downregulation of Wnt and β-catenin proteins in the Wnt signalling pathway, as well as the expression of osteogenesis-related marker molecules such as RUNX2, ALP, and OPN. Conversely, the opposite has been reported when SFRP5 is knocked out, suggesting that SFRP5 may be a key factor involved in the regulation of bone metabolism via the Wnt signalling axis. However, the molecular mechanisms underlying the action of SFRP5-induced OP have yet to be comprehensively elucidated. This review focusses on the molecular structure and function of SFRP5 and the potential molecular mechanisms of the SFRP5-mediated Wnt signalling pathway involved in bone metabolism in OP, providing reasonable evidence for the targeted therapy of SFRP5 for the prevention and treatment of OP.





Similar content being viewed by others
Data Availability
Not applicable.
References
Aspray, T. J., & Hill, T. R. (2019). Osteoporosis and the Ageing Skeleton. SubCellular Biochemistry, 91, 453–476.
Palacios, S. (2022). Medical treatment of osteoporosis. Climacteric, 25, 43–49.
Parveen, B., Parveen, A., & Vohora, D. (2019). Biomarkers of Osteoporosis: An Update. Endocrine, Metabolic & Immune Disorders: Drug Targets, 19, 895–912.
Zhang, L., Zheng, Y. L., Wang, R., Wang, X. Q., & Zhang, H. (2022). Exercise for osteoporosis: A literature review of pathology and mechanism. Frontiers in Immunology, 13, 1005665.
Lei, S. S., Su, J., Zhang, Y., Huang, X. W., Wang, X. P., Huang, M. C., Li, B., & Shou, D. (2021). Benefits andmechanisms of polysaccharides from Chinese medicinal herbs for anti-osteoporosis therapy: A review. Int J BiolMacromol., 193, 1996–2005.
Yavropoulou, M. P., Makras, P., & Anastasilakis, A. D. (2019). Bazedoxifene for the treatment of osteoporosis. Expert Opin Pharmacother, 20, 1201–1210.
Sözen, T., Özışık, L., & Başaran, N. (2017). Anoverview and management of osteoporosis. Eur J Rheumatol, 4, 46–56.
Noh, J. Y., Yang, Y., & Jung, H. (2020). Molecular mechanisms and emerging therapeutics for osteoporosis. International Journal of Molecular Sciences, 21(20), 7623.
Anthamatten, A., & Parish, A. (2019). Clinical update on osteoporosis. Journal of Midwifery and Women’s Health, 64(3), 265–275.
Yu, B., & Wang, C. Y. (2000). Osteoporosis and periodontal diseases-an update on their association and mechanistic links. Periodontology, 89, 99–113.
Adejuyigbe, B., Kallini, J., Chiou, D., & Kallini, J. R. (2023). Osteoporosis: Molecular pathology, diagnostics, and therapeutics. International Journal of Molecular Sciences, 24(19), 14583.
Zhang, J. Y., Zhong, Y. H., Chen, L. M., Zhuo, X. L., Zhao, L. J., & Wang, Y. T. (2023). Recent advance of small-molecule drugs for clinical treatment of osteoporosis: A review. European journal of medicinal chemistry, 259, 115654.
Claudel, M., Jouzeau, J. Y., & Cailotto, F. (2019). Secreted Frizzled-related proteins (sFRPs) in osteo-articular diseases: Much more than simple antagonists of Wnt signaling? FEBS Journal, 286, 4832–4851.
García-García, P., Reyes, R., García-Sánchez, D., Pérez-Campo, F. M., Rodríguez-Rey, J. C., Évora, C., Díaz-Rodríguez, P., & Delgado, A. (2022). Nanoparticle-mediated selective Sfrp-1 silencing enhances bone density in osteoporotic mice. Journal Nanobiotechnology, 20, 462.
Ouchi, N., Higuchi, A., Ohashi, K., Oshima, Y., Gokce, N., Shibata, R., Akasaki, Y., Shimono, A., & Walsh, K. (2010). Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science, 329, 454–457.
He, H. P., & Gu, S. (2021). The PPAR-γ/SFRP5/Wnt/β-catenin signal axis regulates the dexamethasone-induced osteoporosis. Cytokine, 143, 155488.
Chen, H., He, Y., Wu, D., Dai, G., Zhao, C., Huang, W., & Jiang, D. (2017). Bone marrow sFRP5 level is negatively associated with bone formation markers. Osteoporosis International, 28, 1305–1311.
Chen, W., Wu, P., Yu, F., Luo, G., Qing, L., & Tang, J. (2022). HIF-1α regulates bone homeostasis and angiogenesis, participating in the occurrence of bone metabolic diseases. Cells, 11(22), 3552.
Awasthi, H., Mani, D., Singh, D., & Gupta, A. (2018). The underlying pathophysiology and therapeutic approaches for osteoporosis. Medicinal Research Reviews, 38, 2024–2057.
Constanze, B., Popper, B., Aggarwal, B. B., & Shakibaei, M. (2020). Evidence that TNF-β suppresses osteoblast differentiation of mesenchymal stem cells and resveratrol reverses it through modulation of NF-κB, Sirt1 andRunx2. Cell and Tissue Research, 381, 83–98.
Csaki, C., Matis, U., Mobasheri, A., & Shakibaei, M. (2009). Co-culture of canine mesenchymal stem cells with primary bone-derived osteoblasts promotes osteogenic differentiation. Histochemistry and Cell Biology, 131, 251–266.
Lin, P. I., Tai, Y. T., Chan, W. P., Lin, Y. L., Liao, M. H., & Chen, R. M. (2017). Estrogen/ERα signaling axis participates in osteoblast maturation via upregulating chromosomal and mitochondrial complex gene expressions. Oncotarget, 9, 1169–1186.
Chang, J., Wang, Z., Tang, E., Fan, Z., McCauley, L., Franceschi, R., Guan, K., Krebsbach, P. H., & Wang, C. Y. (2009). Inhibition of osteoblastic bone formation by nuclear factor-kappaB. Nature Medicine, 15, 682–689.
Muñoz, M., Robinson, K., & Shibli-Rahhal, A. (2020). Bone Health and Osteoporosis Prevention and Treatment. Clinical Obstetrics and Gynecology, 63, 770–787.
Jilka, R. L. (2003). Biology of the basic multicellular unit and the pathophysiology of osteoporosis. Medical and Pediatric Oncology, 41, 182–185.
Koutaki, D., Michos, A., Bacopoulou, F., & Charmandari, E. (2021). The emerging role of Sfrp5 and Wnt5a in the pathogenesis of obesity: Implications for a healthy diet and lifestyle. Nutrients, 13(7), 2459.
Stuckenholz, C., Lu, L., Thakur, P. C., Choi, T. Y., Shin, D., & Bahary, N. (2013). Sfrp5 modulates both Wnt and BMP signaling and regulates gastrointestinal organogenesis [corrected] in the zebrafish Danio rerio. PLoS One, 8, e62470.
Marinou, K., Christodoulides, C., Antoniades, C., & Koutsilieris, M. (2012). Wnt signaling in cardiovascular physiology. Trends in Endocrinology and Metabolism, 23, 628–636.
Huang, A., & Huang, Y. (2020). Role of Sfrps in cardiovascular disease. Therapeutic Advances in Chronic Disease, 11, 2040622320901990.
Cho, S. W., Her, S. J., Sun, H. J., Choi, O. K., Yang, J. Y., Kim, S. W., Kim, S. Y., & Shin, C. S. (2008). Differential effects of secreted frizzled- related proteins (sFRPs) on osteoblastic differentiation of mouse mesenchymal cells and apoptosis of osteoblasts. Biochemical and Biophysical Research Communications, 367, 399–405.
Bravo, D., Salduz, A., Shogren, K. L., Okuno, M. N., Herrick, J. L., Okuno, S. H., Galindo, M., van Wijnen, A. J., Yaszemski, M. J., & Maran, A. (2018). Decreased local and systemic levels of sFRP3 protein in osteosarcoma patients. Gene, 674, 1–7.
Okamoto, M., Udagawa, N., Uehara, S., Maeda, K., Yamashita, T., Nakamichi, Y., Kato, H., Saito, N., Minami, Y., Takahashi, N., & Kobayashi, Y. (2014). Noncanonical Wnt5a enhances Wnt/β-catenin signaling during osteoblastogenesis. Science and Reports, 4, 4493.
Satoh, W., Matsuyama, M., Takemura, H., Aizawa, S., & Shimono, A. (2008). Sfrp1, Sfrp2, and Sfrp5 regulate the Wnt/beta-catenin and the planar cell polarity pathways during early trunk formation in mouse. Genesis, 46, 92–103.
Maeda, K., Kobayashi, Y., Koide, M., Uehara, S., Okamoto, M., Ishihara, A., Kayama, T., Saito, M., & Marumo, K. (2019). The regulation of bone metabolism and disorders by Wnt signaling. International Journal of Molecular Sciences, 20(22), 5525.
Yao, W., Cheng, Z., Shahnazari, M., Dai, W., Johnson, M. L., & Lane, N. E. (2010). Overexpression of secreted frizzled-related protein 1 inhibits bone formation and attenuates parathyroid hormone bone anabolic effects. Journal of Bone and Mineral Research, 25, 190–199.
Bodine, P. V., Zhao, W., Kharode, Y. P., Bex, F. J., Lambert, A. J., Goad, M. B., Gaur, T., Stein, G. S., Lian, J. B., & Komm, B. S. (2004). The Wnt antagonist secreted frizzled-related protein-1 is a negative regulator of trabecular bone formation in adult mice. Molecular Endocrinology, 18, 1222–1237.
Bodine, P. V., Stauffer, B., Ponce-de-Leon, H., Bhat, R. A., Mangine, A., Seestaller-Wehr, L. M., Moran, R. A., Billiard, J., Fukayama, S., Komm, B. S., Pitts, K., Krishnamurthy, G., Gopalsamy, A., Shi, M., Kern, J. C., Commons, T. J., Woodworth, R. P., Wilson, M. A., Welmaker, G. S., … Moore, W. J. (2009). A small molecule inhibitor of the Wnt antagonist secreted frizzled-related protein-1 stimulates bone formation. Bone, 44, 1063–1068.
Mashhadikhan, M., Kheiri, H., & Dehghanifard, A. (2020). DNA methylation and gene expression of sFRP2, sFRP4, Dkk 1, and Wif1 during osteoblastic differentiation of bone marrow derived mesenchymal stem cells. Journal of Oral Biosciences, 62, 349–356.
Alfaro, M. P., Vincent, A., Saraswati, S., Thorne, C. A., Hong, C. C., Lee, E., & Young, P. P. (2010). sFRP2 suppression of bone morphogenic protein (BMP) and Wnt signaling mediates mesenchymal stem cell (MSC) self-renewal promoting engraftment and myocardial repair. Journal of Biological Chemistry, 285, 35645–35653.
Oshima, T., Abe, M., Asano, J., Hara, T., Kitazoe, K., Sekimoto, E., Tanaka, Y., Shibata, H., Hashimoto, T., Ozaki, S., Kido, S., Inoue, D., & Matsumoto, T. (2005). Myeloma cells suppress bone formation by secreting a soluble Wnt inhibitor, sFRP-2. Blood, 106, 3160–3165.
Yamada, A., Iwata, T., Yamato, M., Okano, T., & Izumi, Y. (2013). Diverse functions of secreted frizzled-related proteins in the osteoblastogenesis of human multipotent mesenchymal stromal cells. Biomaterials, 34, 3270–3278.
Azuma, K., Zhou, Q., & Kubo, K. Y. (2018). Morphological and molecular characterization of the senile osteoporosis in senescence-accelerated mouse prone 6 (SAMP6). Medical Molecular Morphology, 51, 139–146.
Katagiri, W., Osugi, M., Kawai, T., & Hibi, H. (2015). Secreted Frizzled-Related Protein Promotes Bone Regeneration by Human Bone MarrowDerived Mesenchymal Stem Cells. International Journal of Molecular Sciences, 16, 23250–23258.
Parsons, M. J., Tammela, T., & Dow, L. E. (2021). WNT as a Driver and Dependency in Cancer. Cancer Discovery, 11, 2413–2429.
Boudin, E., Fijalkowski, I., Piters, E., & Van Hul, W. (2013). The role of extracellular modulators of canonical Wnt signaling in bone metabolism and diseases. Seminars in Arthritis and Rheumatism, 43, 220–240.
Hayat, R., Manzoor, M., & Hussain, A. (2022). Wnt signaling pathway: A comprehensive review. Cell Biology International, 46, 863–877.
Chien, A. J., Moore, E. C., Lonsdorf, A. S., Kulikauskas, R. M., Rothberg, B. G., Berger, A. J., Major, M. B., Hwang, S. T., Rimm, D. L., & Moon, R. T. (2009). Activated Wnt/beta-catenin signaling in melanoma is associated with decreased proliferation in patient tumors and a murine melanoma model. Proceedings of the National Academy of Sciences, 106, 1193–1198.
Cheng, C. W., Yeh, J. C., Fan, T. P., Smith, S. K., & Charnock-Jones, D. S. (2008). Wnt5a-mediated non-canonical Wnt signalling regulates human endothelial cell proliferation and migration. Biochemical and Biophysical Research Communications, 365, 285–290.
Zhang, X., Wu, M., & Chen, W. (2014). Wnt and the Wnt signaling pathway in bone development and disease. Frontiers in Bioscience (Landmark Edition), 19, 379–407.
Oliva, C. A., Montecinos-Oliva, C., & Inestrosa, N. C. (2018). Wnt Signaling in the Central Nervous System: New Insights in Health and Disease. Progress in Molecular Biology and Translational Science, 153, 81–130.
Taciak, B., Pruszynska, I., Kiraga, L., Bialasek, M., & Krol, M. (2018). Wnt signaling pathway in development and cancer. J Physiol Pharmacol, 69(2), 185–196.
Wang, H., Zhang, R., Wu, X., Chen, Y., Ji, W., Wang, J., Zhang, Y., Xia, Y., Tang, Y., & Yuan, J. (2022). The Wnt signaling pathway in diabetic nephropathy. Frontiers in Cell and Developmental Biology, 9, 701547.
Zhou, Y., Xu, J., Luo, H., Meng, X., Chen, M., & Zhu, D. (2022). Wnt signaling pathway in cancer immunotherapy. Cancer Letters, 525, 84–96.
Wan, Y., Lu, C., Cao, J., Zhou, R., Yao, Y., Yu, J., Zhang, L., Zhao, H., Li, H., Zhao, J., Zhu, X., He, L., Liu, Y., Yao, Z., Yang, X., & Guo, X. (2013). Osteoblastic Wnts differentially regulate bone remodeling and the maintenance of bone marrow mesenchymal stem cells. Bone, 55, 258–267.
Marini, F., Giusti, F., Palmini, G., & Brandi, M. L. (2023). Role of Wnt signaling and sclerostin in bone and as therapeutic targets in skeletal disorders. Osteoporosis International, 34, 213–238.
Zhang, C. J., Zhu, N., Liu, Z., Shi, Z., Long, J., Zu, X. Y., Tang, Z. W., Hu, Z. Y., Liao, D. F., & Qin, L. (2020). Wnt5a/Ror2 pathway contributes to the regulation of cholesterol homeostasis and inflammatory response in atherosclerosis. Biochimica et Biophysica Acta, Molecular and Cell Biology of Lipids, 1865, 158547.
Sharma, M., & Pruitt, K. (2020). Wnt Pathway: an integral hub for developmental and oncogenic signaling networks. International Journal of Molecular Sciences, 21(21), 8018.
Yu, F., Yu, C., Li, F., Zuo, Y., Wang, Y., Yao, L., Wu, C., Wang, C., & Ye, L. (2021). Wnt/β-catenin signaling in cancers and targeted therapies. Signal Transduction and Targeted Therapy, 6, 307.
Rim, E. Y., Clevers, H., & Nusse, R. (2022). The Wnt Pathway: From Signaling Mechanisms to Synthetic Modulators. Annual Review of Biochemistry, 91, 571–598.
Huang, P., Yan, R., Zhang, X., Wang, L., Ke, X., & Qu, Y. (2019). Activating Wnt/β-catenin signaling pathway for disease therapy: Challenges and opportunities. Pharmacology & Therapeutics, 196, 79–90.
Chen, N., & Wang, J. (2018). Wnt/β-catenin signaling and obesity. Frontiers in Physiology , 9, 792.
Martínez-Gil, N., Ugartondo, N., Grinberg, D., & Balcells, S. (2022). Wnt pathway extracellular components and their essential roles in bone homeostasis. Genes (Basel), 13(1), 138.
Choi, R. B., & Robling, A. G. (2021). The Wnt pathway: An important control mechanism in bone’s response to mechanical loading. Bone, 153, 116087.
Maeda, K., Takahashi, N., & Kobayashi, Y. (2013). Roles of Wnt signals in bone resorption during physiological and pathological states. Journal of Molecular Medicine (Berlin, Germany), 91, 15–23.
Lorzadeh, S., Kohan, L., Ghavami, S., & Azarpira, N. (2021). Autophagy and the Wnt signaling pathway: A focus on Wnt/β-catenin signaling. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research, 1868, 118926.
Hernández, A. R., Klein, A. M., & Kirschner, M. W. (2012). Kinetic responses of β-catenin specify the sites of Wnt control. Science, 338, 1337–1340.
Topol, L., Jiang, X., Choi, H., Garrett-Beal, L., Carolan, P. J., & Yang, Y. (2003). Wnt-5a inhibits the canonical Wnt pathway by promoting GSK-3-independent beta-catenin degradation. Journal of Cell Biology, 162, 899–908.
Kikuchi, A., Yamamoto, H., Sato, A., & Matsumoto, S. (2012). Wnt5a: Its signalling, functions and implication in diseases. Acta Psychologica, 204, 17–33.
Sato, A., Yamamoto, H., Sakane, H., Koyama, H., & Kikuchi, A. (2010). Wnt5a regulates distinct signalling pathways by binding to Frizzled2. The EMBO Journal, 29, 41–54.
Martineau, X., Abed, É., Martel-Pelletier, J., Pelletier, J. P., & Lajeunesse, D. (2017). Alteration of Wnt5a expression and of the non-canonical Wnt/PCP and Wnt/PKC-Ca2+ pathways in human osteoarthritis osteoblasts. PLoS ONE, 12, e0180711.
an Amerongen, R., Fuerer, C., Mizutani, M., & Nusse, R. (2012). Wnt5a can both activate and repress Wnt/β-catenin signaling during mouse embryonic development. Developmental Biology, 369, 101–114.
Mi, B., Yan, C., Xue, H., Chen, L., Panayi, A. C., Hu, L., Hu, Y., Cao, F., Sun, Y., Zhou, W., Xiong, Y., & Liu, G. (2020). Inhibition of circulating miR-194-5p reverses osteoporosis through Wnt5a/β-catenin-dependent induction of osteogenic differentiation. Molecular Therapy-Nucleic Acids, 21, 814–823.
Gong, Y., Slee, R. B., Fukai, N., Rawadi, G., Roman-Roman, S., Reginato, A. M., Wang, H., Cundy, T., Glorieux, F. H., Lev, D., Zacharin, M., Oexle, K., Marcelino, J., Suwairi, W., Heeger, S., Sabatakos, G., Apte, S., Adkins, W. N., Allgrove, J., & Arslan-Kirchner, M. (2001). LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell, 107, 513–523.
Little, R. D., Carulli, J. P., Del Mastro, R. G., Dupuis, J., Osborne, M., Folz, C., Manning, S. P., Swain, P. M., Zhao, S. C., Eustace, B., Lappe, M. M., Spitzer, L., Zweier, S., Braunschweiger, K., Benchekroun, Y., Hu, X., Adair, R., Chee, L., FitzGerald, M. G., … Johnson, M. L. (2002). A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. The American Journal of Human Genetics, 70, 11–19.
Boyden, L. M., Mao, J., Belsky, J., Mitzner, L., Farhi, A., Mitnick, M. A., Wu, D., Insogna, K., & Lifton, R. P. (2002). High bone density due to a mutation in LDL-receptor-related protein 5. New England Journal of Medicine, 346, 1513–1521.
Chang, M. K., Kramer, I., Keller, H., Gooi, J. H., Collett, C., Jenkins, D., Ettenberg, S. A., Cong, F., Halleux, C., & Kneissel, M. (2014). Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity. Journal of Bone and Mineral Research, 29, 29–42.
Qiu, W., Andersen, T. E., Bollerslev, J., Mandrup, S., Abdallah, B. M., & Kassem, M. (2007). Patients with high bone mass phenotype exhibit enhanced osteoblast differentiation and inhibition of adipogenesis of human mesenchymal stem cells. Journal of Bone and Mineral Research, 22, 1720–1731.
Kato, M., Patel, M. S., Levasseur, R., Lobov, I., Chang, B. H., Glass, D. A., 2nd., Hartmann, C., Li, L., Hwang, T. H., Brayton, C. F., Lang, R. A., Karsenty, G., & Chan, L. (2002). Cbfa1-independent decrease in osteoblast proliferation, osteopenia, and persistent embryonic eye vascularization in mice deficient in Lrp5, a Wnt coreceptor. The Journal of Cell Biology, 157, 303–314.
Tang, N., Song, W. X., Luo, J., Luo, X., Chen, J., Sharff, K. A., Bi, Y., He, B. C., Huang, J. Y., Zhu, G. H., & Su, Y. X. (2023). BMP9-induced osteogenic differentiation of mesenchymal progenitors requires functional canonical Wnt/β-catenin signalling. Journal of Cellular and Molecular Medicine, 27, 1155–1156.
Semenov, M. V., Habas, R., Macdonald, B. T., & He, X. (2007). SnapShot: Noncanonical Wnt signaling pathways. Cell, 131, 1378.
Butler, M. T., & Wallingford, J. B. (2017). Planar cell polarity in development and disease. Nature Reviews Molecular Cell Biology, 18, 375–388.
Nikolopoulou, E., Galea, G. L., Rolo, A., Greene, N. D., & Copp, A. J. (2017). Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development, 144, 552–566.
Clark, C. E., Nourse, C. C., & Cooper, H. M. (2012). The tangled web of non-canonical Wnt signalling in neural migration. Neurosignals, 20, 202–220.
VanderVorst, K., Dreyer, C. A., Konopelski, S. E., Lee, H., Ho, H. H., & Carraway, K. L., 3rd. (2019). Wnt/PCP Signaling Contribution to Carcinoma Collective Cell Migration and Metastasis. Cancer Research, 79, 1719–1729.
Habas, R., Kato, Y., & He, X. (2001). Wnt/Frizzled activation of Rho regulates vertebrate gastrulation and requires a novel Formin homology protein Daam1. Cell, 107, 843–854.
Yang, Y., & Mlodzik, M. (2015). Wnt-Frizzled/planar cell polarity signaling: Cellular orientation by facing the wind (Wnt). Annual Review of Cell and Developmental Biology, 31, 623–646.
Sonomoto, K., Yamaoka, K., Oshita, K., Fukuyo, S., Zhang, X., Nakano, K., Okada, Y., & Tanaka, Y. (2012). Interleukin-1β induces differentiation of human mesenchymal stem cells into osteoblasts via the Wnt-5a/receptor tyrosine kinase-like orphan receptor 2 pathway. Arthritis and Rheumatism, 64, 3355–3363.
Kramer, I., Halleux, C., Keller, H., Pegurri, M., Gooi, J. H., Weber, P. B., Feng, J. Q., Bonewald, L. F., & Kneissel, M. (2010). Osteocyte Wnt/beta-catenin signaling is required for normal bone homeostasis. Molecular and Cellular Biology, 30, 3071–3085.
Gu, Q., Tian, H., Zhang, K., Chen, D., Chen, D., Wang, X., & Zhao, J. (2018). Wnt5a/FZD4 Mediates the Mechanical Stretch-Induced Osteogenic Differentiation of Bone Mesenchymal Stem Cells. Cellular Physiology and Biochemistry, 48, 215–226.
Chen, L., Zhao, X., Liang, G., Sun, J., Lin, Z., Hu, R., Chen, P., Zhang, Z., Zhou, L., & Li, Y. (2017). Recombinant SFRP5 protein significantly alleviated intrahepatic inflammation of nonalcoholic steatohepatitis. Nutrition & Metabolism (London), 14, 56.
Liu, L. B., Chen, X. D., Zhou, X. Y., & Zhu, Q. (2018). The Wnt antagonist and secreted frizzled-related protein 5: implications on lipid metabolism, inflammation, and type 2 diabetes mellitus. Bioscience Reports, 38(4), BSR20180011.
Xie, Q., Chen, L., Shan, X., Shan, X., Tang, J., Zhou, F., Chen, Q., Quan, H., Nie, D., Zhang, W., Huang, A. L., & Tang, N. (2014). Epigenetic silencing of SFRP1 and SFRP5 by hepatitis B virus X protein enhances hepatoma cell tumorigenicity through Wnt signaling pathway. International Journal of Cancer, 135, 635–646.
Zheng, Y. (2018). Role and mechanism of sFRP5 in bone tissue changes in high-fat diet mice.
Zou, D. P., Chen, Y. M., Zhang, L. Z., Yuan, X. H., Zhang, Y. J., Inggawati, A., Kieu Nguyet, P. T., Gao, T. W., & Chen, J. (2020). SFRP5 inhibits melanin synthesis of melanocytes in vitiligo by suppressing the Wnt/β-catenin signaling. Genes Dis., 8, 677–688.
Wang, B., Pan, Y., Yang, G., Cui, Z., Yu, W., Liu, H., & Bai, B. (2021). Sfrp5/Wnt5a and leptin/ adiponectin levels in the serum and the periarterial adipose tissue of patients with peripheral arterial occlusive disease. Clinical Biochemistry, 87, 46–51.
Sun, M., Wang, W., Min, L., Chen, C., Li, Q., & Weng, W. (2021). Secreted frizzled-related protein 5 (SFRP5) protects ATDC5 cells against LPS-induced inflammation and apoptosis via inhibiting Wnt5a/JNK pathway. Journal of Orthopaedic Surgery and Research, 16, 129.
Li, Q., Zuo, L. L., Lin, Y. Q., Xu, Y. O., Zhu, J. J., Liao, H. H., Lin, S., Xiong, X. R., & Wang, Y. (2016). Cloning and Expression of SFRP5 in Tibetan Chicken and its Relationship with IMF Deposition. Animal Biotechnology, 27, 231–237.
Shi, Z., Xu, M., Chen, X., Wang, J., Zhao, T., & Zha, D. (2021). The regulatory role of SFRP5/ WNT5A axis in allergic rhinitis through inhibitingJNK pathway activation and lowering mucin generation in human nasal epithelial cells. Experimental and Molecular Pathology, 118, 104591.
Wang, R., Hong, J., Liu, R., Chen, M., Xu, M., Gu, W., Zhang, Y., Ma, Q., Wang, F., Shi, J., Wang, J., Wang, W., & Ning, G. (2014). SFRP5 acts as a mature adipocyte marker but not as a regulator in adipogenesis. Journal of Molecular Endocrinology, 53, 405–415.
Cho, Y. K., Kang, Y. M., Lee, S. E., Lee, Y., Seol, S. M., Lee, W. J., Park, J. Y., & Jung, C. H. (2018). Effect of SFRP5 (Secreted Frizzled-Related Protein 5) on the WNT5A (Wingless-Type Family Member 5A)-Induced Endothelial Dysfunction and Its Relevance With Arterial Stiffness in Human Subjects. Arteriosclerosis, Thrombosis, and Vascular Biology, 38, 1358–1367.
Lojk, J., & Marc, J. (2021). Roles of non-canonical wnt signalling pathways in bone biology. International Journal of Molecular Sciences, 22(19), 10840.
Yang, L., Yang, J., Pan, T., & Zhong, X. (2019). Liraglutide increases bone formation and inhibits bone resorption in rats with glucocorticoid-induced osteoporosis. Journal of Endocrinological Investigation, 42, 1125–1131.
Liu, H., Zhan, Y. L., Luo, G. J., Zou, L. L., Li, Y., & Lu, H. Y. (2020). Liraglutide and insulin have contrary effects on adipogenesis of human adipose-derived stem cells via wnt pathway. Diabetes, Metabolic Syndrome and Obesity, 13, 3075–3087.
Iepsen, E. W., Lundgren, J. R., Hartmann, B., Pedersen, O., Hansen, T., Jørgensen, N. R., Jensen, J. E., Holst, J. J., Madsbad, S., & Torekov, S. S. (2015). GLP-1 Receptor Agonist Treatment Increases Bone Formation and Prevents Bone Loss in Weight-Reduced Obese Women. Journal of Clinical Endocrinology and Metabolism, 100, 2909–2917.
Pereira, M., Jeyabalan, J., Jørgensen, C. S., Hopkinson, M., Al-Jazzar, A., Roux, J. P., Chavassieux, P., Orriss, I. R., Cleasby, M. E., & Chenu, C. (2015). Chronic administration of Glucagon-like peptide-1 receptor agonists improves trabecular bone mass and architecture in ovariectomised mice. Bone, 81, 459–467.
Wu, X., Li, S., Xue, P., & Li, Y. (2017). Liraglutide, a glucagon-like peptide-1 receptor agonist, facilitates osteogenic proliferation and differentiation in MC3T3-E1 cells through phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT), extracellular signal-related kinase (ERK)1/2, and cAMP/protein kinase A (PKA) signaling pathways involving β-catenin. Experimental Cell Research, 360, 281–291.
Chen, K., Wu, R., Mo, B., Yan, X., Shen, D., & Chen, M. (2021). Comparison between liraglutide alone and liraglutide in combination with insulin on osteoporotic rats and their effect on bone mineral density. Journal of Musculoskeletal and Neuronal Interactions, 21, 142–148.
Jiang, C., & Gao, M. (2022). Effects of Lilalutide and Alfacalcidol on Blood Glucose, Bone Metabolism, SFRP5 and IGF-1 Levels in Elderly Type 2 diabetes Patients with Osteoporosis. Clinical Medical Research and Practice., 7, 63–66.
Brennan, T. C., Rizzoli, R., & Ammann, P. (2009). Selective modification of bone quality by PTH, pamidronate, or raloxifene. Journal of Bone and Mineral Research, 24, 800–808.
Yang, F., Jia, Y., Sun, Q., Zheng, C., Liu, C., Wang, W., Du, L., Kang, S., Niu, X., & Li, J. (2020). Raloxifene improves TNF-α-induced osteogenic differentiation inhibition of bone marrow mesenchymal stem cells and alleviates osteoporosis. Experimental and Therapeutic Medicine, 20, 309–314.
Heo, H. A., Park, S., Jeon, Y. S., & Pyo, S. W. (2019). Effect of Raloxifene Administration on Bone Response Around Implant in the Maxilla of Osteoporotic Rats. Implant Dentistry, 28, 272–278.
Gizzo, S., Saccardi, C., Patrelli, T. S., Berretta, R., Capobianco, G., Di Gangi, S., Vacilotto, A., Bertocco, A., Noventa, M., Ancona, E., D’Antona, D., & Nardelli, G. B. (2013). Update on raloxifene: Mechanism of action, clinical efficacy, adverse effects, and contraindications. Obstetrical & Gynecological Survey, 68, 467–481.
Park, S., Heo, H. A., Min, J. S., & Pyo, S. W. (2020). Effect of Raloxifene on Bone Formation Around Implants in the Osteoporotic Rat Maxilla: Histomorphometric and Microcomputed Tomographic Analysis. International Journal of Oral and Maxillofacial Implants, 35, 249–456.
Shen, H. H., Yang, C. Y., Kung, C. W., Chen, S. Y., Wu, H. M., Cheng, P. Y., Lam, K. K., & Lee, Y. M. (2019). Raloxifene inhibits adipose tissue inflammation and adipogenesis through Wnt regulation in ovariectomized rats and 3 T3–L1 cells. Journal of Biomedical Science, 26(1), 1–12.
Funding
This review gets grants from the National Natural Science Foundation of China (Grant No.82060872), the Scientific Research Program of Gansu Chinese Medicine Bureau (Grant GZKP-2023-39, and GZKP-2023-63), Research and Reform Project of Graduate Education and Teaching at Gansu University of Chinese Medicine (Grant 15), Teaching Research and Reform Project of Gansu University of Chinese Medicine (Grant YBXM-202332, and ZHXM-202307), and Gansu Province Higher Education Youth Doctoral Fund Project (Grant No.2022QB-091).
Author information
Authors and Affiliations
Contributions
The conception and design of this manuscript was completed by CY. The first draft of this manuscript was written by FA and JS, and FA, JS are co-first authors. The figures and table of the manuscript were made by WC, JZ, PG, YW, and ZX. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
Not applicable.
Consent to Participate
Not applicable.
Consent for Publication
All authors consent to the publication of the article.
Human and Animal Participants
In addition, this article contains no studies involving human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
An, F., Song, J., Chang, W. et al. Research Progress on the Mechanism of the SFRP-Mediated Wnt Signalling Pathway Involved in Bone Metabolism in Osteoporosis. Mol Biotechnol 66, 975–990 (2024). https://doi.org/10.1007/s12033-023-01018-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-023-01018-0