Abstract
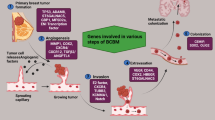
Breast cancer is a multifactorial, heterogeneous disease and the second most frequent cancer amongst women worldwide. Metastasis is one of the most leading causes of death in these patients. Early-stage or locally advanced breast cancer is limited to the breast or nearby lymph nodes. When breast cancer spreads to farther tissues/organs from its original site, it is referred to as metastatic or stage IV breast cancer. Normal breast development is regulated by specific genes and signalling pathways controlling cell proliferation, cell death, cell differentiation and cell motility. Dysregulation of genes involved in various signalling pathways not only leads to the formation of primary tumour but also to the metastasis as well. The metastatic cascade is represented by a multi-step process including invasion of the local tumour cell followed by its entry into the vasculature, exit of malignant cells from the circulation and ultimately their colonization at the distant sites. These stages are referred to as formation of primary tumour, angiogenesis, invasion, intravasation and extravasation, respectively. The major sites of metastasis of breast cancer are the lymph nodes, bone, brain and lung. Only about 28% five-year survival rate has been reported for stage IV breast cancer. Metastasis is a serious concern for breast cancer and therefore, various therapeutic strategies such as tyrosine kinase inhibitors have been developed to target specific dysregulated genes and various signalling pathways involved in different steps of metastasis. In addition, other therapies like hyperbaric oxygen therapy, RNA interference and CRISPR/Cas9 are also being explored as novel strategies to cure the stage IV/metastatic breast cancer. Therefore, the current review has been compiled with an aim to evaluate the genetic basis of stage IV breast cancer with a focus on the molecular mechanisms. In addition, the therapeutic strategies targeting these dysregulated genes involved in various signalling pathways have also been discussed. Genome editing technologies that can target specific genes in the affected areas by making knock-in and knock-out alternations and thereby bring significant treatment outcomes in breast cancer have also been summarized.



Similar content being viewed by others
References
Medeiros B, Allan AL. Molecular mechanisms of breast cancer metastasis to the lung: clinical and experimental perspectives. Int J Mol Sci. 2019;20(9):2272.
Cai Y-D, et al. Identification of genes associated with breast cancer metastasis to bone on a protein–protein interaction network with a shortest path algorithm. J Proteome Res. 2017;16(2):1027–38.
Obenauf AC, Massagué J. Surviving at a distance: organ-specific metastasis. Trends Cancer. 2015;1(1):76–91.
Yazici H, Akin B. Molecular genetics of metastatic breast cancer. In: Tumor progression and metastasis. London: IntechOpen; 2019.
Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–8.
Budczies J, et al. The landscape of metastatic progression patterns across major human cancers. Oncotarget. 2015;6(1):570–83.
Gavrilovic IT, Posner JB. Brain metastases: epidemiology and pathophysiology. J Neurooncol. 2005;75(1):5–14.
Mujoomdar A, et al. Clinical predictors of metastatic disease to the brain from non-small cell lung carcinoma: primary tumor size, cell type, and lymph node metastases. Radiology. 2007;242(3):882–8.
Yousefi M, et al. Lung cancer-associated brain metastasis: molecular mechanisms and therapeutic options. Cell Oncol. 2017;40(5):419–41.
Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8(2):98–101.
Lorusso G, Rüegg C. New insights into the mechanisms of organ-specific breast cancer metastasis. Semin Cancer Biol. 2012;22(3):226–33.
Nicolini A, Carpi A, Rossi G. Cytokines in breast cancer. Cytokine Growth Factor Rev. 2006;17(5):325–37.
Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–59.
Hsing CH, et al. Upregulated IL-19 in breast cancer promotes tumor progression and affects clinical outcome. Clin Cancer Res. 2012;18(3):713–25.
Lu X, Kang Y. Organotropism of breast cancer metastasis. J Mammary Gland Biol Neoplasia. 2007;12(2–3):153–62.
Chiang AC, Massagué J. Molecular basis of metastasis. N Engl J Med. 2008;359(26):2814–23.
Tauro M, Lynch CC. Cutting to the chase: how matrix metalloproteinase-2 activity controls breast-cancer-to-bone metastasis. Cancers. 2018;10(6):185.
Bell R, Barraclough R, Vasieva O. Gene expression meta-analysis of potential metastatic breast cancer markers. Curr Mol Med. 2017;17(3):200–10.
Blanco MA, Kang Y. Signaling pathways in breast cancer metastasis - novel insights from functional genomics. Breast Cancer Res. 2011;13(2):206.
Feng Y, et al. Breast cancer development and progression: risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis. 2018;5(2):77–106.
Welch DR, Steeg PS, Rinker-Schaeffer CW. Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res. 2000;2(6):408–16.
Cominetti MR, Altei WF, Selistre-de-Araujo HS. Metastasis inhibition in breast cancer by targeting cancer cell extravasation. Breast Cancer (Dove Medical Press). 2019;11:165–78.
Li Z, et al. Methylation-associated silencing of MicroRNA-335 contributes tumor cell invasion and migration by interacting with RASA1 in gastric cancer. Am J Cancer Res. 2014;4(6):648–62.
Zhao W, et al. Mutations of BRAF and KRAS in gastric cancer and their association with microsatellite instability. Int J Cancer. 2004;108(1):167–9.
Zandvakili I, et al. Loss of RhoA exacerbates, rather than dampens, oncogenic K-Ras induced lung adenoma formation in mice. PLoS ONE. 2015;10(6):e0127923.
Kim H, Choi JA, Kim JH. Ras promotes transforming growth factor-β (TGF-β)-induced epithelial-mesenchymal transition via a leukotriene B4 receptor-2-linked cascade in mammary epithelial cells. J Biol Chem. 2014;289(32):22151–60.
Gilhooly EM, Rose DP. The association between a mutated ras gene and cyclooxygenase-2 expression in human breast cancer cell lines. Int J Oncol. 1999;15(2):267–70.
Kim MS, et al. p38 kinase is a key signaling molecule for H-Ras-induced cell motility and invasive phenotype in human breast epithelial cells. Cancer Res. 2003;63(17):5454–61.
Shin I, et al. H-Ras-specific activation of Rac-MKK3/6-p38 pathway: its critical role in invasion and migration of breast epithelial cells. J Biol Chem. 2005;280(15):14675–83.
Yoon SO, Shin S, Mercurio AM. Ras stimulation of E2F activity and a consequent E2F regulation of integrin alpha6beta4 promote the invasion of breast carcinoma cells. Cancer Res. 2006;66(12):6288–95.
Ono M, et al. WISP-1/CCN4 regulates osteogenesis by enhancing BMP-2 activity. J Bone Miner Res. 2011;26(1):193–208.
Sieuwerts AM, et al. Progressive APOBEC3B mRNA expression in distant breast cancer metastases. PLoS ONE. 2017;12(1):e0171343.
Maruyama W, et al. Classical NF-κB pathway is responsible for APOBEC3B expression in cancer cells. Biochem Biophys Res Commun. 2016;478(3):1466–71.
Chizzolini C, et al. Th2 cell membrane factors in association with IL-4 enhance matrix metalloproteinase-1 (MMP-1) while decreasing MMP-9 production by granulocyte-macrophage colony-stimulating factor-differentiated human monocytes. J Immunol. 2000;164(11):5952–60.
Lagow EL, Carson DD. Synergistic stimulation of MUC1 expression in normal breast epithelia and breast cancer cells by interferon-gamma and tumor necrosis factor-alpha. J Cell Biochem. 2002;86(4):759–72.
Banno T, Gazel A, Blumenberg M. Effects of tumor necrosis factor-alpha (TNF alpha) in epidermal keratinocytes revealed using global transcriptional profiling. J Biol Chem. 2004;279(31):32633–42.
Tang LY, et al. Quantitative phosphoproteome profiling of Wnt3a-mediated signaling network: indicating the involvement of ribonucleoside-diphosphate reductase M2 subunit phosphorylation at residue serine 20 in canonical Wnt signal transduction. Mol Cell Proteomics. 2007;6(11):1952–67.
Madu CO, et al. Angiogenesis in breast cancer progression, diagnosis, and treatment. J Cancer. 2020;11(15):4474–94.
Clark KL, et al. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature. 1993;364(6436):412–20.
Korver W, et al. The human TRIDENT/HFH-11/FKHL16 gene: structure, localization, and promoter characterization. Genomics. 1997;46(3):435–42.
Wang IC, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25(24):10875–94.
Laoukili J, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005;7(2):126–36.
Pilarsky C, et al. Identification and validation of commonly overexpressed genes in solid tumors by comparison of microarray data. Neoplasia. 2004;6(6):744–50.
Bektas N, et al. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. 2008;8:42.
Chen YJ, et al. A conserved phosphorylation site within the forkhead domain of FoxM1B is required for its activation by cyclin-CDK1. J Biol Chem. 2009;284(44):30695–707.
Fu Z, et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10(9):1076–82.
Park HJ, et al. FoxM1, a critical regulator of oxidative stress during oncogenesis. Embo J. 2009;28(19):2908–18.
Chaudhary A, et al. TEM8/ANTXR1 blockade inhibits pathological angiogenesis and potentiates tumoricidal responses against multiple cancer types. Cancer Cell. 2012;21(2):212–26.
Chen D, et al. ANTXR1, a stem cell-enriched functional biomarker, connects collagen signaling to cancer stem-like cells and metastasis in breast cancer. Can Res. 2013;73(18):5821–33.
Kang Y, et al. Breast cancer bone metastasis mediated by the Smad tumor suppressor pathway. Proc Natl Acad Sci USA. 2005;102(39):13909–14.
Caldon CE, Musgrove EA. Distinct and redundant functions of cyclin E1 and cyclin E2 in development and cancer. Cell Div. 2010;5:2.
Zanin R, et al. HMGA1 promotes breast cancer angiogenesis supporting the stability, nuclear localization and transcriptional activity of FOXM1. J Exp Clin Cancer Res. 2019;38(1):313.
Shah SN, et al. HMGA1: a master regulator of tumor progression in triple-negative breast cancer cells. PLoS ONE. 2013;8(5):e63419–e63419.
Martin, T.A., et al., Cancer invasion and metastasis: molecular and cellular perspective. In Madame Curie Bioscience Database [Internet]. 2013, Landes Bioscience.
Vervoort SJ, et al. Global transcriptional analysis identifies a novel role for SOX4 in tumor-induced angiogenesis. Elife. 2018;7:e27706.
Kaur T, et al. Identification of functional SNPs in human LGALS3 gene by in silico analyses. Egypt J Med Hum Genet. 2017;18(4):321–8.
Ruvolo PP. Galectin 3 as a guardian of the tumor microenvironment. Biochim Biophys Acta. 2016;1863(3):427–37.
Ochieng J, et al. Modulation of the biological functions of galectin-3 by matrix metalloproteinases. Biochem Biophys Acta. 1998;1379(1):97–106.
Balan V, et al. Racial disparity in breast cancer and functional germ line mutation in galectin-3 (rs4644): a pilot study. Can Res. 2008;68(24):10045–50.
Pachmayr E, Treese C, Stein U. Underlying mechanisms for distant metastasis - molecular biology. Visc Med. 2017;33(1):11–20.
Xie HY, Shao ZM, Li DQ. Tumor microenvironment: driving forces and potential therapeutic targets for breast cancer metastasis. Chin J Cancer. 2017;36(1):36.
Chen Y, Olopade OI. MYC in breast tumor progression. Expert Rev Anticancer Ther. 2008;8(10):1689–98.
Tuo Y, An N, Zhang M. Feature genes in metastatic breast cancer identified by MetaDE and SVM classifier methods. Mol Med Rep. 2018;17(3):4281–90.
Barber E, et al. Orbital metastases from breast cancer with BRCA2 mutation: a case report and literature review. Case Rep Oncol. 2018;11(2):360–4.
Godet I, Gilkes DM. BRCA1 and BRCA2 mutations and treatment strategies for breast cancer. Integr Cancer Sci Ther. 2017. https://doi.org/10.15761/ICST.1000228.
Tsai MS, et al. Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies. Cancer Res. 2000;60(20):5603–7.
Awolaran O, Brooks SA, Lavender V. Breast cancer osteomimicry and its role in bone specific metastasis; an integrative, systematic review of preclinical evidence. Breast. 2016;30:156–71.
Khoshakhlagh M, et al. Therapeutic potential of pharmacological TGF-β signaling pathway inhibitors in the pathogenesis of breast cancer. Biochem Pharmacol. 2019;164:17–22.
Chen L-C, et al. Human breast cancer cell metastasis is attenuated by lysyl oxidase inhibitors through down-regulation of focal adhesion kinase and the paxillin-signaling pathway. Breast Cancer Res Treat. 2012;134(3):989–1004.
Erler JT, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440(7088):1222–6.
Müller A, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50–6.
Singh AK, et al. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013;24(1):41–9.
Mareel M, et al. E-cadherin/catenin/cytoskeleton complex: a regulator of cancer invasion. J Cell Physiol. 1997;173(2):271–4.
Vermeulen SJ, et al. Transition from the noninvasive to the invasive phenotype and loss of alpha-catenin in human colon cancer cells. Cancer Res. 1995;55(20):4722–8.
Frixen UH, et al. E-cadherin-mediated cell-cell adhesion prevents invasiveness of human carcinoma cells. J Cell Biol. 1991;113(1):173–85.
Martin T, et al. Cancer invasion and metastasis: molecular and cellular perspective. Austin: Landes Bioscience; 2013. p. 135–68.
Chiang SPH, Cabrera RM, Segall JE. Tumor cell intravasation. Am J Physiol Cell Physiol. 2016;311(1):C1–14.
Qian Y, Shi L, Luo Z. Long non-coding RNAs in cancer: implications for diagnosis, prognosis, and therapy. Front Med. 2020;7(902):612393.
Patsialou A, et al. Autocrine CSF1R signaling mediates switching between invasion and proliferation downstream of TGFβ in claudin-low breast tumor cells. Oncogene. 2015;34(21):2721–31.
Giampieri S, et al. Localized and reversible TGFβ signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11(11):1287–96.
Keklikoglou I, et al. MicroRNA-520/373 family functions as a tumor suppressor in estrogen receptor negative breast cancer by targeting NF-κB and TGF-β signaling pathways. Oncogene. 2012;31(37):4150–63.
Dangi-Garimella S, et al. Raf kinase inhibitory protein suppresses a metastasis signalling cascade involving LIN28 and let-7. EMBO J. 2009;28(4):347–58.
Frankenberger C, et al. Metastasis suppressors regulate the tumor microenvironment by blocking recruitment of prometastatic tumor-associated macrophages. Can Res. 2015;75(19):4063–73.
Deryugina EI, et al. Unexpected effect of matrix metalloproteinase down-regulation on vascular intravasation and metastasis of human fibrosarcoma cells selected in vivo for high rates of dissemination. Can Res. 2005;65(23):10959–69.
Bekes EM, et al. Tumor-recruited neutrophils and neutrophil TIMP-free MMP-9 regulate coordinately the levels of tumor angiogenesis and efficiency of malignant cell intravasation. Am J Pathol. 2011;179(3):1455–70.
Deryugina EI, Quigley JP. Tumor angiogenesis: MMP-mediated induction of intravasation-and metastasis-sustaining neovasculature. Matrix Biol. 2015;44:94–112.
Chabottaux V, et al. Membrane-type 4 matrix metalloproteinase (MT4-MMP) induces lung metastasis by alteration of primary breast tumour vascular architecture. J Cell Mol Med. 2009;13(9B):4002–13.
Chou CH, et al. MMP-9 from sublethally irradiated tumor promotes Lewis lung carcinoma cell invasiveness and pulmonary metastasis. Oncogene. 2012;31(4):458–68.
Kubala MH, DeClerck YA. The plasminogen activator inhibitor-1 paradox in cancer: a mechanistic understanding. Cancer Metastasis Rev. 2019;38(3):483–92.
Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell. 2017;168(4):670–91.
Ahmed H, AlSadek DMM. Galectin-3 as a potential target to prevent cancer metastasis. Clinical medicine insights. Oncology. 2015;9:113–21.
Wu K, et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–54.
Brosnan EM, Anders CK. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Ann Transl Med. 2018;6(9):163.
Dunn LK, et al. Hypoxia and TGF-β drive breast cancer bone metastases through parallel signaling pathways in tumor cells and the bone microenvironment. PLoS ONE. 2009;4(9):e6896.
Wendel C, et al. CXCR4/CXCL12 participate in extravasation of metastasizing breast cancer cells within the liver in a rat model. PLoS ONE. 2012;7(1):e30046.
Padua D, et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77.
Reymond N, d’Água BB, Ridley AJ. Crossing the endothelial barrier during metastasis. Nat Rev Cancer. 2013;13(12):858–70.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20):6243s–9s.
Tahara RK, et al. Bone metastasis of breast cancer. Adv Exp Med Biol. 2019;1152:105–29.
Parfitt A. Targeted and nontargeted bone remodeling: relationship to basic multicellular unit origination and progression. Bone. 2002;30(1):5–7.
Xiong J, O’Brien CA. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Miner Res. 2012;27(3):499–505.
Fu Q, Manolagas SC, O’Brien CA. Parathyroid hormone controls receptor activator of NF-κB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol. 2006;26(17):6453–68.
Tatsumi S, et al. Targeted ablation of osteocytes induces osteoporosis with defective mechanotransduction. Cell Metab. 2007;5(6):464–75.
Mathot L, Stenninger J. Behavior of seeds and soil in the mechanism of metastasis: a deeper understanding. Cancer Sci. 2012;103(4):626–31.
Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10(2):169–80.
Coleman, R.E. and J.J. Seaman. The role of zoledronic acid in cancer: clinical studies in the treatment and prevention of bone metastases. in Seminars in oncology. 2001. Elsevier.
Chirgwin JM, Guise TA. Molecular mechanisms of tumor-bone interactions in osteolytic metastases. Crit Rev Eukaryot Gene Exp. 2000;10(2):159–78.
Onishi T, et al. Future directions of bone-targeted therapy for metastatic breast cancer. Nat Rev Clin Oncol. 2010;7(11):641.
Guise TA. The vicious cycle of bone metastases. J Musculoskelet Neuronal Interact. 2002;2(6):570–2.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64.
Theriault RL, Theriault RL. Biology of bone metastases. Cancer Control. 2012;19(2):92–101.
Simonet W, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89(2):309–19.
Clines G, Guise T. Hypercalcaemia of malignancy and basic research on mechanisms responsible for osteolytic and osteoblastic metastasis to bone. Endocr Relat Cancer. 2005;12(3):549–83.
Yoo B, Fuchs BC, Medarova Z. New directions in the study and treatment of metastatic cancer. Front Oncol. 2018;8:258–258.
Fazilaty H, et al. Crosstalk between breast cancer stem cells and metastatic niche: emerging molecular metastasis pathway? Tumor Biol. 2013;34(4):2019–30.
Chelouche Lev D, Price JE. Therapeutic intervention with breast cancer metastasis. Crit Rev Eukaryot Gene Expr. 2002;12(2):137–50.
Kawalec P, Łopuch S, Mikrut A. Effectiveness of targeted therapy in patients with previously untreated metastatic breast cancer: a systematic review and meta-analysis. Clin Breast Cancer. 2015;15(2):90-100.e1.
Pottier C, et al. Tyrosine kinase inhibitors in cancer: breakthrough and challenges of targeted therapy. Cancers. 2020;12(3):731.
Butti R, et al. Receptor tyrosine kinases (RTKs) in breast cancer: signaling, therapeutic implications and challenges. Mol Cancer. 2018;17(1):34.
Yang X, Wu D, Yuan S. Tyrosine kinase inhibitors in the combination therapy of HER2 positive breast cancer. Technol Cancer Res Treat. 2020;19:1533033820962140.
Pawson T. Regulation and targets of receptor tyrosine kinases. Eur J Cancer. 2002;38(Suppl 5):S3-10.
Escudier B, Gore M. Axitinib for the management of metastatic renal cell carcinoma. Drugs R D. 2011;11(2):113–26.
Rugo HS, et al. Randomized, placebo-controlled, double-blind, phase II study of axitinib plus docetaxel versus docetaxel plus placebo in patients with metastatic breast cancer. J Clin Oncol. 2011;29(18):2459–65.
Wilmes LJ, et al. AG-013736, a novel inhibitor of VEGF receptor tyrosine kinases, inhibits breast cancer growth and decreases vascular permeability as detected by dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging. 2007;25(3):319–27.
Gunnarsson O, et al. Evaluating the safety and efficacy of axitinib in the treatment of advanced renal cell carcinoma. Cancer Manag Res. 2015;7:65–73.
Facchini G, et al. Second line therapy with axitinib after only prior sunitinib in metastatic renal cell cancer: Italian multicenter real world SAX study final results. J Transl Med. 2019;17(1):296.
Hu-Lowe DD, et al. Nonclinical antiangiogenesis and antitumor activities of axitinib (AG-013736), an oral, potent, and selective inhibitor of vascular endothelial growth factor receptor tyrosine kinases 1, 2, 3. Clin Cancer Res. 2008;14(22):7272–83.
Wehland M, et al. Target-based anti-angiogenic therapy in breast cancer. Curr Pharm Des. 2012;18(27):4244–57.
Isaacs C, et al. Phase I/II study of sorafenib with anastrozole in patients with hormone receptor positive aromatase inhibitor resistant metastatic breast cancer. Breast Cancer Res Treat. 2011;125(1):137–43.
Dattachoudhury S, et al. Sorafenib inhibits proliferation, migration and invasion of breast cancer cells. Oncology. 2020;98(7):478–86.
Reddy S, Raffin M, Kaklamani V. Targeting angiogenesis in metastatic breast cancer. Oncologist. 2012;17(8):1014–26.
Zafrakas M, Papasozomenou P, Emmanouilides C. Sorafenib in breast cancer treatment: a systematic review and overview of clinical trials. World J Clin Oncol. 2016;7(4):331–6.
Tolaney SM, et al. Cabozantinib for metastatic breast carcinoma: results of a phase II placebo-controlled randomized discontinuation study. Breast Cancer Res Treat. 2016;160(2):305–12.
Yakes FM, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–308.
Zhu C, Wei Y, Wei X. AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer. 2019;18(1):153.
Mundhenke C, Strauss A, Schem C. Significance of tyrosine kinase inhibitors in the treatment of metastatic breast cancer. Breast care (Basel, Switzerland). 2009;4(6):373–8.
Burstein HJ, et al. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26(11):1810–6.
Wang D, et al. Sunitinib facilitates metastatic breast cancer spreading by inducing endothelial cell senescence. Breast Cancer Res. 2020;22(1):103.
Chaffer CL, Weinberg RA. How does multistep tumorigenesis really proceed? Cancer Discov. 2015;5(1):22–4.
Hare SH, Harvey AJ. MTOR function and therapeutic targeting in breast cancer. Am J Cancer Res. 2017;7(3):383–404.
Masoud V, Pagès G. Targeted therapies in breast cancer: new challenges to fight against resistance. World J Clin Oncol. 2017;8(2):120–34.
Vinayak S, Carlson RW. mTOR inhibitors in the treatment of breast cancer. Oncology (Williston Park). 2013;27(1):38–44.
Steelman LS, et al. The therapeutic potential of mTOR inhibitors in breast cancer. Br J Clin Pharmacol. 2016;82(5):1189–212.
Miller K, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357(26):2666–76.
Torrisi R, et al. Preoperative bevacizumab combined with letrozole and chemotherapy in locally advanced ER- and/or PgR-positive breast cancer: clinical and biological activity. Br J Cancer. 2008;99(10):1564–71.
Reardon DA, Cheresh D. Cilengitide: a prototypic integrin inhibitor for the treatment of glioblastoma and other malignancies. Genes Cancer. 2011;2(12):1159–65.
Bäuerle T, et al. Cilengitide inhibits progression of experimental breast cancer bone metastases as imaged noninvasively using VCT, MRI and DCE-MRI in a longitudinal in vivo study. Int J Cancer. 2011;128(10):2453–62.
Gutheil JC, et al. Targeted antiangiogenic therapy for cancer using vitaxin: a humanized monoclonal antibody to the integrin ανβ3. Clin Cancer Res. 2000;6(8):3056–61.
Castañeda-Gill JM, Vishwanatha JK. Antiangiogenic mechanisms and factors in breast cancer treatment. J Carcinogen. 2016;15:1–1.
Falardeau P, et al. Neovastat, a naturally occurring multifunctional antiangiogenic drug, in phase III clinical trials. Semin Oncol. 2001;28(6):620–5.
Weber MH, Lee J, Orr FW. The effect of Neovastat (AE-941) on an experimental metastatic bone tumor model. Int J Oncol. 2002;20(2):299–303.
Gill AL, Bell CN. Hyperbaric oxygen: its uses, mechanisms of action and outcomes. QJM. 2004;97(7):385–95.
Moen I, Stuhr LE. Hyperbaric oxygen therapy and cancer–a review. Target Oncol. 2012;7(4):233–42.
Yttersian Sletta K, et al. Oxygen-dependent regulation of tumor growth and metastasis in human breast cancer xenografts. PLoS ONE. 2017;12(8):e0183254–e0183254.
Mast JM, Kuppusamy P. Hyperoxygenation as a therapeutic supplement for treatment of triple negative breast cancer. Front Oncol. 2018;8:527.
Ceci C, et al. Experimental evidence of the antitumor, antimetastatic and antiangiogenic activity of ellagic acid. Nutrients. 2018;10(11):1756.
Saleem A, et al. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. fruit. J Ethnopharmacol. 2002;81(3):327–36.
Shi L, et al. Ellagic acid enhances the efficacy of PI3K inhibitor GDC-0941 in breast cancer cells. Curr Mol Med. 2015;15(5):478–86.
Wang N, et al. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat. 2012;134(3):943–55.
Holen I, Coleman RE. Bisphosphonates as treatment of bone metastases. Curr Pharm Des. 2010;16(11):1262–71.
Rogers MJ, et al. Cellular and molecular mechanisms of action of bisphosphonates. Cancer Interdiscip Int J Am Cancer Soc. 2000;88(S12):2961–78.
van der Pluijm G, et al. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Investig. 1996;98(3):698–705.
Boissier S, et al. Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Can Res. 2000;60(11):2949–54.
Daubiné F, et al. Antitumor effects of clinical dosing regimens of bisphosphonates in experimental breast cancer bone metastasis. J Natl Cancer Inst. 2007;99(4):322–30.
Senaratne S, et al. Bisphosphonates induce apoptosis in human breast cancer cell lines. Br J Cancer. 2000;82(8):1459–68.
Maniar A, et al. Human γδ T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116(10):1726–33.
Benzaïd I, et al. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vγ9Vδ2 T-cell chemotaxis and cytotoxicity in vivo. Can Res. 2011;71(13):4562–72.
Syddall SP, Ottewell PD, Holen I. Combined therapies of bone disease with bisphosphonates. Curr Pharm Des. 2010;16(27):2988–97.
Lipton A, et al. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25(28):4431–7.
Canon JR, et al. Inhibition of RANKL blocks skeletal tumor progression and improves survival in a mouse model of breast cancer bone metastasis. Clin Exp Metastasis. 2008;25(2):119–29.
Stopeck AT, et al. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28(35):5132–9.
Mossing MC, Record MT Jr. Upstream operators enhance repression of the lac promoter. Science. 1986;233(4766):889–92.
Henry DH, et al. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29(9):1125–32.
Lipton A, et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–92.
Henry D, et al. Delaying skeletal-related events in a randomized phase 3 study of denosumab versus zoledronic acid in patients with advanced cancer: an analysis of data from patients with solid tumors. Support Care Cancer. 2014;22(3):679–87.
Wang X, et al. Comparison of the efficacy and safety of denosumab versus bisphosphonates in breast cancer and bone metastases treatment: a meta-analysis of randomized controlled trials. Oncol Lett. 2014;7(6):1997–2002.
Wong MH, Stockler MR, Pavlakis N. Bisphosphonates and other bone agents for breast cancer. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD003474.pub4.
Bora RS, et al. RNA interference therapeutics for cancer: challenges and opportunities (review). Mol Med Rep. 2012;6(1):9–15.
Tao W, et al. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell. 2005;8(1):49–59.
Santel A, et al. Atu027 prevents pulmonary metastasis in experimental and spontaneous mouse metastasis models. Clin Cancer Res. 2010;16(22):5469–80.
Liu X. Targeting polo-like kinases: a promising therapeutic approach for cancer treatment. Transl Oncol. 2015;8(3):185–95.
Takai N, et al. Polo-like kinases (Plks) and cancer. Oncogene. 2005;24(2):287–91.
Guan R, et al. Small interfering RNA-mediated Polo-like kinase 1 depletion preferentially reduces the survival of p53-defective, oncogenic transformed cells and inhibits tumor growth in animals. Cancer Res. 2005;65(7):2698–704.
Spänkuch B, et al. Cancer inhibition in nude mice after systemic application of U6 promoter-driven short hairpin RNAs against PLK1. J Natl Cancer Inst. 2004;96(11):862–72.
Yang G, et al. Inhibition of breast and ovarian tumor growth through multiple signaling pathways by using retrovirus-mediated small interfering RNA against Her-2/neu gene expression. J Biol Chem. 2004;279(6):4339–45.
Liang Z, et al. Silencing of CXCR4 blocks breast cancer metastasis. Can Res. 2005;65(3):967–71.
Huynh A, Madu CO, Lu Y. siRNA: a promising new tool for future breast cancer therapy. Oncomedicine. 2018;3:74–81.
Tian X, et al. CRISPR/Cas9: an evolving biological tool kit for cancer biology and oncology. NPJ Precis Oncol. 2019;3:8.
Padayachee J, Singh M. Therapeutic applications of CRISPR/Cas9 in breast cancer and delivery potential of gold nanomaterials. Nanobiomedicine. 2020;7:1849543520983196.
Liu J, et al. Differential effects of estrogen receptor β isoforms on glioblastoma progression. Cancer Res. 2018;78(12):3176–89.
Pranavathiyani G, et al. Integrated transcriptome interactome study of oncogenes and tumor suppressor genes in breast cancer. Genes & diseases. 2018;6(1):78–87.
Wang H, Sun W. CRISPR-mediated targeting of HER2 inhibits cell proliferation through a dominant negative mutation. Cancer Lett. 2017;385:137–43.
Guo P, et al. Therapeutic genome editing of triple-negative breast tumors using a noncationic and deformable nanolipogel. Proc Natl Acad Sci U S A. 2019;116(37):18295–303.
Chen D, et al. Super enhancer inhibitors suppress MYC driven transcriptional amplification and tumor progression in osteosarcoma. Bone Res. 2018;6:11.
Schuijers J, et al. Transcriptional dysregulation of MYC reveals common enhancer-docking mechanism. Cell Rep. 2018;23(2):349–60.
Fallah Y, et al. MYC-driven pathways in breast cancer subtypes. Biomolecules. 2017;7(3):53.
Sun H, et al. SIRT4 acts as a tumor suppressor in gastric cancer by inhibiting cell proliferation, migration, and invasion. Onco Targets Ther. 2018;11:3959–68.
Jiang C, Lin X, Zhao Z. Applications of CRISPR/Cas9 technology in the treatment of lung cancer. Trends Mol Med. 2019;25(11):1039–49.
Xu K, et al. MFN2 suppresses cancer progression through inhibition of mTORC2/Akt signaling. Sci Rep. 2017;7:41718.
Jin K, Pandey NB, Popel AS. Crosstalk between stromal components and tumor cells of TNBC via secreted factors enhances tumor growth and metastasis. Oncotarget. 2017;8(36):60210.
Zhang S, et al. Suppression of protein tyrosine phosphatase N23 predisposes to breast tumorigenesis via activation of FYN kinase. Genes Dev. 2017;31(19):1939–57.
Yang M, et al. Impact of CXCR4 and CXCR7 knockout by CRISPR/Cas9 on the function of triple-negative breast cancer cells. Onco Targets Ther. 2019;12:3849–58.
Hazafa A, et al. CRISPR/Cas9: A powerful genome editing technique for the treatment of cancer cells with present challenges and future directions. Life Sci. 2020;263:118525–118525.
Høye AM, et al. Tumor endothelial marker 8 promotes cancer progression and metastasis. Oncotarget. 2018;9(53):30173–88.
Welch DR, Steeg PS, Rinker-Schaeffer CW. Molecular biology of breast cancer metastasis. Genetic regulation of human breast carcinoma metastasis. Breast Cancer Res. 2000;2(6):1–9.
Calvo A, et al. Identification of VEGF-regulated genes associated with increased lung metastatic potential: functional involvement of tenascin-C in tumor growth and lung metastasis. Oncogene. 2008;27(40):5373–84.
Gilkes DM, Semenza GL. Role of hypoxia-inducible factors in breast cancer metastasis. Future Oncol. 2013;9(11):1623–36.
Lee JY, et al. Gene expression profiling of breast cancer brain metastasis. Sci Rep. 2016;6(1):1–10.
Estiar MA, Mehdipour P. ATM in breast and brain tumors: a comprehensive review. Cancer Biol Med. 2018;15(3):210.
Sirkisoon SR, et al. EGFR and HER2 signaling in breast cancer brain metastasis. Front Biosci. 2016;8:245.
Bektas N, et al. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. 2008;8(1):1–9.
Salhia, B., et al., Integrated genomic and epigenomic analysis of breast cancer brain metastasis. PLoS ONE, 2014. 9(1): p. e85448.
Wang X, Lin Y. Tumor necrosis factor and cancer, buddies or foes? Acta Pharmacol Sin. 2008;29(11):1275–88.
Mustafa DA, et al. T lymphocytes facilitate brain metastasis of breast cancer by inducing guanylate-binding protein 1 expression. Acta Neuropathol. 2018;135(4):581–99.
Fan W, et al. RUVBL1-ITFG1 interaction is required for collective invasion in breast cancer. Biochem Biophys Acta. 2017;1861(7):1788–800.
Zanin R, et al. HMGA1 promotes breast cancer angiogenesis supporting the stability, nuclear localization and transcriptional activity of FOXM1. J Exp Clin Cancer Res. 2019;38(1):1–23.
Cominetti MR, Altei WF, Selistre-de-Araujo HS. Metastasis inhibition in breast cancer by targeting cancer cell extravasation. Breast Cancer. 2019;11:165.
Liu S, et al. Vascular endothelial growth factor plays a critical role in the formation of the pre-metastatic niche via prostaglandin E2. Oncol Rep. 2014;32(6):2477–84.
Malik FA, et al. Effect of expressional alteration of KAI1 on breast cancer cell growth, adhesion, migration and invasion. Cancer Genomics-Proteomics. 2009;6(4):205–13.
Blanco MA, Kang Y. Signaling pathways in breast cancer metastasis-novel insights from functional genomics. Breast Cancer Res. 2011;13(2):1–9.
Liu S, et al. MAP2K4 interacts with Vimentin to activate the PI3K/AKT pathway and promotes breast cancer pathogenesis. Aging (Albany NY). 2019;11(22):10697.
Prud’homme GJ, Glinka Y. Neuropilins are multifunctional coreceptors involved in tumor initiation, growth, metastasis and immunity. Oncotarget. 2012;3(9):921.
Bai J, et al. BRG1 is a prognostic marker and potential therapeutic target in human breast cancer. PLoS ONE. 2013;8(3):e59772.
Lee H, et al. TMEM2 Is a SOX4-regulated gene that mediates metastatic migration and invasion in breast cancer. Can Res. 2016;76(17):4994–5005.
Yesilkanal AE, Rosner MR. Raf kinase inhibitory protein (RKIP) as a metastasis suppressor: regulation of signaling networks in cancer. Crit Rev Oncog. 2014;19(6):447–54.
Chao YL, Shepard CR, Wells A. Breast carcinoma cells re-express E-cadherin during mesenchymal to epithelial reverting transition. Mol Cancer. 2010;9(1):179.
Bevilacqua E, Frankenberger CA, Rosner MR. RKIP Suppresses breast cancer metastasis to the bone by regulating stroma-associated genes. Int J Breast Cancer. 2012;2012:124704.
Malinowsky K, et al. UPA and PAI-1-related signaling pathways differ between primary breast cancers and lymph node metastases. Transl Oncol. 2012;5(2):98–104.
Mahauad-Fernandez WD, et al. BST-2 promotes survival in circulation and pulmonary metastatic seeding of breast cancer cells. Sci Rep. 2018;8(1):17608.
Pedrosa RMSM, et al. Breast cancer brain metastasis: molecular mechanisms and directions for treatment. Neuro Oncol. 2018;20(11):1439–49.
Harris RE, Casto BC, Harris ZM. Cyclooxygenase-2 and the inflammogenesis of breast cancer. World J Clin Oncol. 2014;5(4):677–92.
Strilic B, Offermanns S. Intravascular survival and extravasation of tumor cells. Cancer Cell. 2017;32(3):282–93.
Wu X, Chen S, Lu C. Amyloid precursor protein promotes the migration and invasion of breast cancer cells by regulating the MAPK signaling pathway. Int J Mol Med. 2020;45(1):162–74.
Fu Q, Manolagas SC, O’Brien CA. Parathyroid hormone controls receptor activator of NF-kappaB ligand gene expression via a distant transcriptional enhancer. Mol Cell Biol. 2006;26(17):6453–68.
Vesuna F, Bergman Y, Raman V. Genomic pathways modulated by Twist in breast cancer. BMC Cancer. 2017;17(1):52.
Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12(20 Pt 2):6243s–9s.
Acknowledgements
We acknowledge Central University of Punjab for providing infrastructure and facility. Indian Council of Medical Research (ICMR), New Delhi, is acknowledged for Research Associateship 2019-4834/CMB-BMS to P.S. and Council of Scientific and Industrial Research (CSIR), New Delhi, for Junior Research Fellowship (JRF) to Y.C. for Ph.D.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Chhichholiya, Y., Suman, P., Singh, S. et al. The genomic architecture of metastasis in breast cancer: focus on mechanistic aspects, signalling pathways and therapeutic strategies. Med Oncol 38, 95 (2021). https://doi.org/10.1007/s12032-021-01547-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-021-01547-1