Abstract
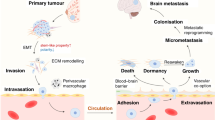
Breast cancer (BC) is one of the most prevalent types of cancer in women. Despite advancement in early detection and efficient treatment, recurrence and metastasis continue to pose a significant risk to the life of BC patients. Brain metastasis (BM) reported in 17–20 percent of BC patients is considered as a major cause of mortality and morbidity in these patients. BM includes various steps from primary breast tumor to secondary tumor formation. Various steps involved are primary tumor formation, angiogenesis, invasion, extravasation, and brain colonization. Genes involved in different pathways have been reported to be associated with BC cells metastasizing to the brain. ADAM8 gene, EN1 transcription factor, WNT, and VEGF signaling pathway have been associated with primary breast tumor; MMP1, COX2, XCR4, PI3k/Akt, ERK and MAPK pathways in angiogenesis; Noth, CD44, Zo-1, CEMIP, S0X2 and OLIG2 are involved in invasion, extravasation and colonization, respectively. In addition, the blood–brain barrier is also a key factor in BM. Dysregulation of cell junctions, tumor microenvironment and loss of function of microglia leads to BBB disruption ultimately resulting in BM. Various therapeutic strategies are currently used to control the BM in BC. Oncolytic virus therapy, immune checkpoint inhibitors, mTOR-PI3k inhibitors and immunotherapy have been developed to target various genes involved in BM in BC. In addition, RNA interference (RNAi) and CRISPR/Cas9 are novel interventions in the field of BCBM where research to validate these and clinical trials are being carried out. Gaining a better knowledge of metastasis biology is critical for establishing better treatment methods and attaining long-term therapeutic efficacies against BC. The current review has been compiled with an aim to evaluate the role of various genes and signaling pathways involved in multiple steps of BM in BC. The therapeutic strategies being used currently and the novel ones being explored to control BM in BC have also been discussed at length.




Similar content being viewed by others
Data availability
Not Applicable.
References
Yadav UP, Ansari AJ, Arora S, Joshi G, Singh T, Kaur H, et al. Design, synthesis and anticancer activity of 2-arylimidazo [1, 2-a] pyridinyl-3-amines. Bioorg Chem. 2022;118: 105464.
Yadav UP, Singh T, Kumar P, Sharma P, Kaur H, Sharma S, et al. Metabolic adaptations in cancer stem cells. Front Oncol. 2020;10:1010.
Sung, H., J. Ferlay, R.L. Siegel, M. Laversanne, I. Soerjomataram, A. Jemal, et al (2021) GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 71: 3 209–249.
Parsa N. Environmental factors inducing human cancers. Iran J Public Health. 2012;41(11):1–9.
Li X, Wang J. Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int J Biol Sci. 2020;16(12):2014.
Martelotto LG, Ng CK, Piscuoglio S, Weigelt B, Reis-Filho JS. Breast cancer intra-tumor heterogeneity. Breast Cancer Res. 2014;16(3):1–11.
Chhichholiya Y, Suman P, Singh S, Munshi A. The genomic architecture of metastasis in breast cancer: focus on mechanistic aspects. Signal Pathways Therap Strat. 2021;38(8):95.
Liu L, Zhang Y, Lu J. The roles of long noncoding RNAs in breast cancer metastasis. Cell Death Dis. 2020;11(9):1–14.
Hosonaga M, Saya H, Arima Y. Molecular and cellular mechanisms underlying brain metastasis of breast cancer. Cancer Metastasis Rev. 2020;39(3):711–20.
Kanchan RK, Siddiqui JA, Mahapatra S, Batra SK, Nasser MW. microRNAs orchestrate pathophysiology of breast cancer brain metastasis: advances in therapy. Mol Cancer. 2020;19(1):1–16.
Wanleenuwat P, Iwanowski P. Metastases to the central nervous system: molecular basis and clinical considerations. J Neurol Sci. 2020;412: 116755.
Arvanitis CD, Ferraro GB, Jain RK. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat Rev Cancer. 2020;20(1):26–41.
Ulasov I, Borovjagin A, Fares J, Yakushov S, Malin D, Timashev P, et al. MicroRNA 345 (miR345) regulates KISS1-E-cadherin functional interaction in breast cancer brain metastases. Cancer Lett. 2020;481:24–31.
Fares J, Kanojia D, Rashidi A, Ulasov I, Lesniak MS. Genes that mediate metastasis across the blood–brain barrier. Trends in cancer. 2020;6(8):660–76.
Martin AM, Cagney DN, Catalano PJ, Warren LE, Bellon JR, Punglia RS, et al. Brain metastases in newly diagnosed breast cancer: a population-based study. JAMA Oncol. 2017;3(8):1069–77.
Niikura N, Hayashi N, Masuda N, Takashima S, Nakamura R, Watanabe K-I, et al. Treatment outcomes and prognostic factors for patients with brain metastases from breast cancer of each subtype: a multicenter retrospective analysis. Breast Cancer Res Treat. 2014;147(1):103–12.
Watase C, Shiino S, Shimoi T, Noguchi E, Kaneda T, Yamamoto Y, et al. Breast cancer brain metastasis—overview of disease state, treatment options and future perspectives. Cancers. 2021;13(5):1078.
Lin NU, Claus E, Sohl J, Razzak AR, Arnaout A, Winer EP. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer. 2008;113(10):2638–45.
Yao Y, Chu Y. Risk factors for distant metastasis of patients with primary triple-negative. Breast Cancer. 2019;39:6.
Yomtoubian S, Lee SB, Verma A, Izzo F, Markowitz G, Choi H, et al. Inhibition of EZH2 catalytic activity selectively targets a metastatic subpopulation in triple-negative breast cancer. Cell Rep. 2020;30(3):755-770.e6.
Peluffo G, Subedee A, Harper NW. EN1 is a transcriptional dependency in triple-negative. Breast Cancer Assoc Brain Metast. 2019;79(16):4173–83.
Sartorius CA, Hanna CT, Gril B, Cruz H, Serkova NJ, Huber KM, et al. Estrogen promotes the brain metastatic colonization of triple negative breast cancer cells via an astrocyte-mediated paracrine mechanism. Oncogene. 2016;35(22):2881–92.
Morissette M, Le Saux M, D’astous M, Jourdain S, Al Sweidi S, Morin N, et al. Contribution of estrogen receptors alpha and beta to the effects of estradiol in the brain. J Ster Biochem Molec Biol. 2008;108(3):327–38.
García-Ovejero D, Veiga S, García-Segura LM, Doncarlos LL. Glial expression of estrogen and androgen receptors after rat brain injury. J Compar Neurol. 2002;450(3):256–71.
Contreras-Zárate MJ, Day NL, Ormond DR, Borges VF, Tobet S, Gril B, et al. Estradiol induces BDNF/TrkB signaling in triple-negative breast cancer to promote brain metastases. Oncogene. 2019;38(24):4685–99.
Choy C, Ansari KI, Neman J, Hsu S, Duenas MJ, Li H, et al. Cooperation of neurotrophin receptor TrkB and Her2 in breast cancer cells facilitates brain metastases. Breast Cancer Res. 2017;19(1):1–11.
Zhou L, Zhou W, Zhang H, Hu Y, Yu L, Zhang Y, et al. Progesterone suppresses triple-negative breast cancer growth and metastasis to the brain via membrane progesterone receptor α. Int J Mol Med. 2017;40(3):755–61.
Krystal cascetta, A.t. (2021). Primary invasive breast cancer. https://bestpractice.bmj.com/topics/en-us/716.
Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408(6810):307–10.
Powell E, Piwnica-Worms D, Piwnica-Worms H. Contribution of p53 to metastasis. Cancer Discov. 2014;4(4):405–14.
Brosh R, Rotter V. When mutants gain new powers: news from the mutant p53 field. Nat Rev Cancer. 2009;9(10):701–13.
Wei C-L, Wu Q, Vega VB, Chiu KP, Ng P, Zhang T, et al. A global map of p53 transcription-factor binding sites in the human genome. Cell. 2006;124(1):207–19.
Pharoah PD, Day NE, Caldas C. Somatic mutations in the p53 gene and prognosis in breast cancer: a meta-analysis. Br J Cancer. 1999;80(12):1968–73.
Olivier M, Langerød A, Carrieri P, Bergh J, Klaar S, Eyfjord J, et al. The clinical value of somatic TP53 gene mutations in 1,794 patients with breast cancer. Clin Cancer Res. 2006;12(4):1157–67.
Lee JY, Park K, Lim SH, Kim HS, Yoo KH, Jung KS, et al. Mutational profiling of brain metastasis from breast cancer: matched pair analysis of targeted sequencing between brain metastasis and primary breast cancer. Oncotarget. 2015;6(41):43731–42.
Lo Nigro C, Vivenza D, Monteverde M, Lattanzio L, Gojis O, Garrone O, et al. High frequency of complex TP53 mutations in CNS metastases from breast cancer. Br J Cancer. 2012;106(2):397–404.
Koller G, Schlomann U, Golfi P, Ferdous T, Naus S, Bartsch JW. ADAM8/MS2/CD156, an emerging drug target in the treatment of inflammatory and invasive pathologies. Curr Pharm Des. 2009;15(20):2272–81.
Conrad C, Götte M, Schlomann U, Roessler M, Pagenstecher A, Anderson P, et al. ADAM8 expression in breast cancer derived brain metastases: Functional implications on MMP-9 expression and transendothelial migration in breast cancer cells. Int J Cancer. 2018;142(4):779–91.
Mohammed RAA, Green A, El-Shikh S, Paish E, Ellis I, Martin S. Prognostic significance of vascular endothelial cell growth factors-A,-C and-D in breast cancer and their relationship with angio-and lymphangiogenesis. Br J Cancer. 2007;96(7):1092–100.
Hawinkels LJ, Zuidwijk K, Verspaget HW, de Jonge-Muller ES, van Duijn W, Ferreira V, et al. VEGF release by MMP-9 mediated heparan sulphate cleavage induces colorectal cancer angiogenesis. Eur J Cancer. 2008;44(13):1904–13.
Linderholm B, Hellborg H, Johansson U, Elmberger G, Skoog L, Lehtiö J, et al. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann Oncol. 2009;20(10):1639–46.
Romagnoli M, Mineva ND, Polmear M, Conrad C, Srinivasan S, Loussouarn D, et al. ADAM8 expression in invasive breast cancer promotes tumor dissemination and metastasis. EMBO Mol Med. 2014;6(2):278–94.
Vandermeersch S, Vanbeselaere J, Delannoy CP, Drolez A, Mysiorek C, Guérardel Y, et al. Accumulation of GD1α ganglioside in MDA-MB-231 breast cancer cells expressing ST6GalNAc V. Molecules. 2015;20(4):6913–24.
Drolez A, Vandenhaute E, Delannoy CP, Dewald JH, Gosselet F, Cecchelli R, et al. ST6GALNAC5 expression decreases the interactions between breast cancer cells and the human blood-brain barrier. Int J Mol Sci. 2016;17(8):1309.
Bos PD, Zhang XH, Nadal C, Shu W, Gomis RR, Nguyen DX, et al. Genes that mediate breast cancer metastasis to the brain. Nature. 2009;459(7249):1005–9.
Guenzi E, Töpolt K, Lubeseder-Martellato C, Jörg A, Naschberger E, Benelli R, et al. The guanylate binding protein-1 GTPase controls the invasive and angiogenic capability of endothelial cells through inhibition of MMP-1 expression. EMBO J. 2003;22(15):3772–82.
Honkala AT, Tailor D, Malhotra SV. Guanylate-binding protein 1: an emerging target in inflammation and cancer. Front Immunol. 2020;10:3139.
Mustafa DAM, Pedrosa RMSM, Smid M, van der Weiden M, de Weerd V, Nigg AL, et al. T lymphocytes facilitate brain metastasis of breast cancer by inducing Guanylate-Binding Protein 1 expression. Acta Neuropathol. 2018;135(4):581–99.
Alves dos Santos M, Smidt MP. En1 and Wnt signaling in midbrain dopaminergic neuronal development. Neural Develop. 2011;6(1):1–15.
Peluffo G, Subedee A, Harper NW, Kingston N, Jovanović B, Flores F, et al. EN1 is a transcriptional dependency in triple-negative breast cancer associated with brain metastasis. Can Res. 2019;79(16):4173–83.
Bachar-Dahan L, Goltzmann J, Yaniv A, Gazit A. Engrailed-1 negatively regulates β-Catenin transcriptional activity by destabilizing β-catenin via a glycogen synthase kinase-3β–independent pathway. Mol Biol Cell. 2006;17(6):2572–80.
Arese M, Serini G, Bussolino F. Nervous vascular parallels: axon guidance and beyond. Int J Dev Biol. 2011;55(4–5):439–45.
Alakbarzade V, Hameed A, Quek DQ, Chioza BA, Baple EL, Cazenave-Gassiot A, et al. A partially inactivating mutation in the sodium-dependent lysophosphatidylcholine transporter MFSD2A causes a non-lethal microcephaly syndrome. Nat Genet. 2015;47(7):814–7.
Guemez-Gamboa A, Nguyen LN, Yang H, Zaki MS, Kara M, Ben-Omran T, et al. Inactivating mutations in MFSD2A, required for omega-3 fatty acid transport in brain, cause a lethal microcephaly syndrome. Nat Genet. 2015;47(7):809–13.
Tiwary S, Morales JE, Kwiatkowski SC, Lang FF, Rao G, McCarty JH. Metastatic brain tumors disrupt the blood-brain barrier and alter lipid metabolism by inhibiting expression of the endothelial cell fatty acid transporter Mfsd2a. Sci Rep. 2018;8(1):8267.
Ben-Zvi A, Lacoste B, Kur E, Andreone BJ, Mayshar Y, Yan H, et al. Mfsd2a is critical for the formation and function of the blood-brain barrier. Nature. 2014;509(7501):507–11.
O’Brown NM, Megason SG, Gu C. Suppression of transcytosis regulates zebrafish blood-brain barrier function. Elife. 2019;8:47326.
Andreone BJ, Chow BW, Tata A, Lacoste B, Ben-Zvi A, Bullock K, et al. Blood-brain barrier permeability is regulated by lipid transport-dependent suppression of caveolae-mediated transcytosis. Neuron. 2017;94(3):581-594.e5.
Zetter BR. Angiogenesis and tumor metastasis. Annu Rev Med. 1998;49:407–24.
Zuazo-Gaztelu I, Casanovas O. Unraveling the role of angiogenesis in cancer ecosystems. Front. Oncol. 2018;8:248.
Muz B, de la Puente P, Azab F, Azab AK. The role of hypoxia in cancer progression, angiogenesis, metastasis, and resistance to therapy. Hypoxia (Auckland, NZ). 2015;3:83–92.
Tobar LE, Farnsworth RH, Stacker SA. Brain vascular microenvironments in cancer metastasis. Biomolecules. 2022;12(3):401.
Rodrigues G, Hoshino A, Kenific CM, Matei IR, Steiner L, Freitas D, et al. Tumour exosomal CEMIP protein promotes cancer cell colonization in brain metastasis. Nat Cell Biol. 2019;21(11):1403–12.
Wu K, Fukuda K, Xing F, Zhang Y, Sharma S, Liu Y, et al. Roles of the cyclooxygenase 2 matrix metalloproteinase 1 pathway in brain metastasis of breast cancer. J Biol Chem. 2015;290(15):9842–54.
Poola I, DeWitty RL, Marshalleck JJ, Bhatnagar R, Abraham J, Leffall LD. Identification of MMP-1 as a putative breast cancer predictive marker by global gene expression analysis. Nat Med. 2005;11(5):481–3.
Pei D. Matrix metalloproteinases target protease-activated receptors on the tumor cell surface. Cancer Cell. 2005;7(3):207–8.
McCawley LJ, Matrisian LM. Matrix metalloproteinases: they’re not just for matrix anymore! Curr Opin Cell Biol. 2001;13(5):534–40.
Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114(1):33–45.
Blackburn JS, Brinckerhoff CE. Matrix metalloproteinase-1 and thrombin differentially activate gene expression in endothelial cells via PAR-1 and promote angiogenesis. Am J Pathol. 2008;173(6):1736–46.
Liu H, Kato Y, Erzinger SA, Kiriakova GM, Qian Y, Palmieri D, et al. The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer. 2012;12:583–583.
Brandt, M.F.L.S.F.L.J., Sleisenger and Fordtran's Gastrointestinal and Liver Disease. 11 ed, ed. M. Mark Feldman, Lawrence S. Friedman, MD and Lawrence J. Brandt, MD. Vol. 2. 2020, Massachusetts: elviser. 2488.
Majumder M, Dunn L, Liu L, Hasan A, Vincent K, Brackstone M, et al. COX-2 induces oncogenic micro RNA miR655 in human breast cancer. Sci Rep. 2018;8(1):327.
Sugimoto Y, Narumiya S. Prostaglandin E receptors. J Biol Chem. 2007;282(16):11613–7.
Fujino H, Xu W, Regan JW. Prostaglandin E2 induced functional expression of early growth response factor-1 by EP4, but not EP2, prostanoid receptors via the phosphatidylinositol 3-kinase and extracellular signal-regulated kinases. J Biol Chem. 2003;278(14):12151–6.
Ma X, Holt D, Kundu N, Reader J, Goloubeva O, Take Y, et al. A prostaglandin E (PGE) receptor EP4 antagonist protects natural killer cells from PGE(2)-mediated immunosuppression and inhibits breast cancer metastasis. Oncoimmunology. 2013;2(1): e22647.
Majumder M, Xin X, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014;105(9):1142–51.
Reel B, Sala-Newby GB, Huang WC, Newby AC. Diverse patterns of cyclooxygenase-independent metalloproteinase gene regulation in human monocytes. Br J Pharmacol. 2011;163(8):1679–90.
Lee KY, Kim Y-J, Yoo H, Lee SH, Park JB, Kim HJ. Human brain endothelial cell-derived COX-2 facilitates extravasation of breast cancer cells across the blood–brain barrier. Anticancer Res. 2011;31(12):4307–13.
Moss LAS, Jensen-Taubman S, Stetler-Stevenson WG. Matrix metalloproteinases: changing roles in tumor progression and metastasis. Am J Pathol. 2012;181(6):1895–9.
Xu C, Zhao H, Chen H, Yao Q. CXCR4 in breast cancer: oncogenic role and therapeutic targeting. Drug Des Devel Ther. 2015;9:4953–64.
Luther SA, Bidgol A, Hargreaves DC, Schmidt A, Xu Y, Paniyadi J, et al. Differing activities of homeostatic chemokines CCL19, CCL21, and CXCL12 in lymphocyte and dendritic cell recruitment and lymphoid neogenesis. J Immunol. 2002;169(1):424–33.
Shi Y, Riese DJ, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front. Pharmacol. 2020;11:574667.
Kang H, Watkins G, Parr C, Douglas-Jones A, Mansel RE, Jiang WG. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005;7(4):R402.
Fares J, Kanojia D, Rashidi A, Ulasov I, Lesniak MS. Genes that mediate metastasis across the blood-brain barrier. Trends Cancer. 2020;6(8):660–76.
Würth R, Bajetto A, Harrison JK, Barbieri F, Florio T. CXCL12 modulation of CXCR4 and CXCR7 activity in human glioblastoma stem-like cells and regulation of the tumor microenvironment. Front. Cell. Neurosci. 2014;8:144.
Gong X, Hou Z, Endsley MP, Gronseth EI, Rarick KR, Jorns JM, et al. Interaction of tumor cells and astrocytes promotes breast cancer brain metastases through TGF-β2/ANGPTL4 axes. npj Prec Oncol. 2019;3(1):24.
Zhu P, Tan MJ, Huang R-L, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2−:H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19(3):401–15.
Padua D, Zhang XH, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77.
Akhtar M, Haider A, Rashid S, Al-Nabet A. Paget’s “seed and soil” theory of cancer metastasis: an idea whose time has come. Adv Anat Pathol. 2019;26(1):69–74.
Suman P, Chhichholiya Y, Kaur P, Ghosh S, Munshi A. Long non-coding RNAs involved in different steps of cancer metastasis. Clin. Transl. Oncol. 2022;1–17.
Ostrand-Rosenberg S, Fenselau C. Myeloid-derived suppressor cells: immune-suppressive cells that impair antitumor immunity and are sculpted by their environment. J Immunol (Baltim Md 1950). 2018;200(2):422–31.
Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci. 2014;34(24):8186–96.
Miyamoto N, Maki T, Shindo A, Liang AC, Maeda M, Egawa N, et al. Astrocytes promote oligodendrogenesis after white matter damage via brain-derived neurotrophic factor. J Neurosci Off J Soc Neurosci. 2015;35(41):14002–8.
Ding H, Roncari L, Shannon P, Wu X, Lau N, Karaskova J, et al. Astrocyte-specific expression of activated p21-ras results in malignant astrocytoma formation in a transgenic mouse model of human gliomas. Can Res. 2001;61(9):3826–36.
Huang Y, Rane SG. Potassium channel induction by the Ras/Raf signal transduction cascade. J Biol Chem. 1994;269(49):31183–9.
Khaitan D, Sankpal UT, Weksler B, Meister EA, Romero IA, Couraud P-O, et al. Role of KCNMA1 gene in breast cancer invasion and metastasis to brain. BMC Cancer. 2009;9(1):1–11.
Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Can Res. 2006;66(3):1517–25.
Mungamuri SK, Yang X, Thor AD, Somasundaram K. Survival signaling by Notch1: mammalian target of rapamycin (mTOR)–dependent inhibition of p53. Can Res. 2006;66(9):4715–24.
Lee CW, Simin K, Liu Q, Plescia J, Guha M, Khan A, et al. A functional Notch–survivin gene signature in basal breast cancer. Breast Cancer Res. 2008;10(6):1–12.
Dickson BC, Mulligan AM, Zhang H, Lockwood G, O’Malley FP, Egan SE, et al. High-level JAG1 mRNA and protein predict poor outcome in breast cancer. Mod Pathol. 2007;20(6):685–93.
McGowan PM, Simedrea C, Ribot EJ, Foster PJ, Palmieri D, Steeg PS, et al. Notch1 inhibition alters the CD44hi/CD24lo population and reduces the formation of brain metastases from breast cancer. Mol Cancer Res. 2011;9(7):834–44.
Fazakas C, Wilhelm I, Nagyőszi P, Farkas AE, Haskó J, Molnár J, et al. Transmigration of melanoma cells through the blood-brain barrier: role of endothelial tight junctions and melanoma-released serine proteases. PLoS ONE. 2011;6(6): e20758.
Lee T-H, Avraham HK, Jiang S, Avraham S. Vascular endothelial growth factor modulates the transendothelial migration of MDA-MB-231 breast cancer cells through regulation of brain microvascular endothelial cell permeability. J Biol Chem. 2003;278(7):5277–84.
Mine S, Fujisaki T, Kawahara C, Tabata T, Iida T, Yasuda M, et al. Hepatocyte growth factor enhances adhesion of breast cancer cells to endothelial cells in vitro through up-regulation of CD44. Exp Cell Res. 2003;288(1):189–97.
Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Can Res. 2004;64(16):5702–11.
Avraham HK, Jiang S, Fu Y, Nakshatri H, Ovadia H, Avraham S. Angiopoietin-2 mediates blood–brain barrier impairment and colonization of triple-negative breast cancer cells in brain. J Pathol. 2014;232(3):369–81.
Lee, B.-C., T.-H. Lee, S. Avraham, and H.K. Avraham, Involvement of the Chemokine Receptor CXCR4 and Its Ligand Stromal Cell-Derived Factor 1α in Breast Cancer Cell Migration Through Human Brain Microvascular Endothelial Cells11NIH grant NS39558 (S. Avraham), the Susan G. Komen Fellowship (S. Avraham), the Milheim Foundation (S. Avraham), CA97153 (H. Avraham), and K18 PAR-02–069 (H. Avraham). Note: This work was done during the term of an established investigatorship from the American Heart Association (H. Avraham). This article is dedicated to Charlene Engelhard for her continuing friendship and support for our research program. Molecular Cancer Research, 2004. 2(6): p. 327–338.
Szmulewitz, R., J. Taylor, and C. Rinker-Schaffer, Metastatic Colonization, in Encyclopedia of Cancer, M. Schwab, Editor. 2011, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 2271–2273.
Fidler IJ. The biology of brain metastasis: challenges for therapy. Cancer J. 2015;21(4):284–93.
Lee JY, Park K, Lee E, Ahn T, Jung HH, Lim SH, et al. Gene expression profiling of breast cancer brain metastasis. Sci Rep. 2016;6(1):28623.
Zhou M, Hua W, Sun Y. Cell migration inducing hyaluronidase 1 promotes growth and metastasis of papillary thyroid carcinoma. Bioengineered. 2022;13(5):11822–11831. https://doi.org/10.1080/21655979.2022.2074110
Burri PH, Hlushchuk R, Djonov V. Intussusceptive angiogenesis: its emergence, its characteristics, and its significance. Dev Dyn. 2004;231(3):474–88.
Dimova I, Hlushchuk R, Makanya A, Styp-Rekowska B, Ceausu A, Flueckiger S, et al. Inhibition of Notch signaling induces extensive intussusceptive neo-angiogenesis by recruitment of mononuclear cells. Angiogenesis. 2013;16(4):921–37.
Giacomini A, Ackermann M, Belleri M, Coltrini D, Nico B, Ribatti D, et al. Brain angioarchitecture and intussusceptive microvascular growth in a murine model of Krabbe disease. Angiogenesis. 2015;18(4):499–510.
Gene, N. SOX Sry box transription factor 2. [english] 2022 [cited 2022 27th march]; description about SOX2 gene and its function]. Available from: https://www.who.int/news-room/fact-sheets/detail/breast-cancer.
Xiao W, Zheng S, Xie X, Li X, Zhang L, Yang A, et al. SOX2 promotes brain metastasis of breast cancer by upregulating the expression of FSCN1 and HBEGF. Molecular therapy oncolytics. 2020;17:118–29.
Zhang S, Cui W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World J Stem Cells. 2014;6(3):305–11.
Ligon KL, Alberta JA, Kho AT, Weiss J, Kwaan MR, Nutt CL, et al. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J Neuropathol Exp Neurol. 2004;63(5):499–509.
Park ES, Kim SJ, Kim SW, Yoon SL, Leem SH, Kim SB, et al. Cross-species hybridization of microarrays for studying tumor transcriptome of brain metastasis. Proc Natl Acad Sci U S A. 2011;108(42):17456–61.
Eckhardt BL, Parker BS, van Laar RK, Restall CM, Natoli AL, Tavaria MD, et al. Genomic analysis of a spontaneous model of breast cancer metastasis to bone reveals a role for the extracellular matrix. Mol Cancer Res. 2005;3(1):1–13.
Magnussen SN, Toraskar J, Wilhelm I, Hasko J, Figenschau SL, Molnar J, et al. Nephronectin promotes breast cancer brain metastatic colonization via its integrin-binding domains. Sci Rep. 2020;10(1):12237.
Iliff JJ, Goldman SA, Nedergaard M. Implications of the discovery of brain lymphatic pathways. The Lancet Neurology. 2015;14(10):977–9.
Feldman, M., L.S. Friedman, and L.J. Brandt, Sleisenger and Fordtran's gastrointestinal and liver disease E-book: pathophysiology, diagnosis, management. 2020: Elsevier health sciences.
Majumder M, Xin X, Liu L, Girish GV, Lala PK. Prostaglandin E2 receptor EP 4 as the common target on cancer cells and macrophages to abolish angiogenesis, lymphangiogenesis, metastasis, and stem-like cell functions. Cancer Sci. 2014;105(9):1142–51.
Shi Y, Riese DJ, Shen J. The role of the CXCL12/CXCR4/CXCR7 chemokine axis in cancer. Front. Pharmacol. 2020;11:574667.
Gong X, Hou Z, Endsley MP, Gronseth EI, Rarick KR, Jorns JM, et al. Interaction of tumor cells and astrocytes promotes breast cancer brain metastases through TGF-β2/ANGPTL4 axes. NPJ Prec Oncol. 2019;3(1):1–9.
Zhu P, Tan MJ, Huang R-L, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2−: H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19(3):401–15.
Padua D, Zhang XH-F, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFβ primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133(1):66–77.
Guan Z, Lan H, Cai X, Zhang Y, Liang A, Li J. Blood–brain barrier, cell junctions, and tumor microenvironment in brain metastases, the biological prospects and dilemma in therapies. Front. Cell Dev. Biol. 2021;9:722917.
Berghoff AS, Lassmann H, Preusser M, Höftberger R. Characterization of the inflammatory response to solid cancer metastases in the human brain. Clin Exp Metas. 2013;30(1):69–81.
Lee JY, Park K, Lee E, Ahn T, Jung HH, Lim SH, et al. Gene expression profiling of breast cancer brain metastasis. Sci Rep. 2016;6(1):1–10.
Xiao W, Zheng S, Xie X, Li X, Zhang L, Yang A, et al. SOX2 promotes brain metastasis of breast cancer by upregulating the expression of FSCN1 and HBEGF. Molec Therapy-Oncol. 2020;17:118–29.
Arshad F, Wang L, Sy C, Avraham S, Avraham HK. Blood-brain barrier integrity and breast cancer metastasis to the brain. Pathol Res Int. 2010;2011:920509–920509.
Bos PD, Nguyen DX, Massagué J. Modeling metastasis in the mouse. Curr Opin Pharmacol. 2010;10(5):571–7.
Winkler EA, Bell RD, Zlokovic BV. Central nervous system pericytes in health and disease. Nat Neurosci. 2011;14(11):1398–405.
Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16(1):1–13.
Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190(3):310–29.
Yano S, Shinohara H, Herbst RS, Kuniyasu H, Bucana CD, Ellis LM, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Can Res. 2000;60(17):4959–67.
Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin. Dev. Immunol. 2011;842849. https://doi.org/10.1155/2011/842849
Bobrie A, Krumeich S, Reyal F, Recchi C, Moita LF, Seabra MC, et al. Rab27a supports exosome-dependent and-independent mechanisms that modify the tumor microenvironment and can promote tumor progression. Can Res. 2012;72(19):4920–30.
Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, et al. Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 2012;18(6):883–91.
Serlin Y, Shelef I, Knyazer B, Friedman A. Anatomy and physiology of the blood–brain barrier. In: Seminars in cell & developmental biology (2015). (Vol. 38, pp. 2–6). Academic Press.
Seelbach M, Chen L, Powell A, Choi YJ, Zhang B, Hennig B, et al. Polychlorinated biphenyls disrupt blood–brain barrier integrity and promote brain metastasis formation. Environ Health Perspect. 2010;118(4):479–84.
Godinho-Pereira J, Garcia AR, Figueira I, Malhó R, Brito MA. Behind Brain Metastases Formation: Cellular and Molecular Alterations and Blood-Brain Barrier Disruption. Int J Mol Sci. 2021;22(13):7057.
Dejana E. Endothelial cell–cell junctions: happy together. Nat Rev Mol Cell Biol. 2004;5(4):261–70.
Feng S, Cen J, Huang Y, Shen H, Yao L, Wang Y, et al. Matrix metalloproteinase-2 and-9 secreted by leukemic cells increase the permeability of blood-brain barrier by disrupting tight junction proteins. PLoS ONE. 2011;6(8): e20599.
Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma-astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–8.
Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11(2):87–99.
Chen Q, Boire A, Jin X, Valiente M, Er EE, Lopez-Soto A, et al. Carcinoma–astrocyte gap junctions promote brain metastasis by cGAMP transfer. Nature. 2016;533(7604):493–8.
Zou Y, Watters A, Cheng N, Perry CE, Xu K, Alicea GM, et al. Polyunsaturated fatty acids from astrocytes activate PPARγ signaling in cancer cells to promote brain metastasis. Cancer Discov. 2019;9(12):1720–35.
Pozzobon T, Goldoni G, Viola A, Molon B. CXCR4 signaling in health and disease. Immunol Lett. 2016;177:6–15.
Kawaguchi N, Zhang T-T, Nakanishi T. Involvement of CXCR4 in normal and abnormal development. Cells. 2019;8(2):185.
Berghoff AS, Fuchs E, Ricken G, Mlecnik B, Bindea G, Spanberger T, et al. Density of tumor-infiltrating lymphocytes correlates with extent of brain edema and overall survival time in patients with brain metastases. Oncoimmunology. 2016;5(1): e1057388.
Fujimoto T, Nakagawa S, Morofuji Y, Watanabe D, Ujifuku K, Horie N, et al. Pericytes suppress brain metastasis from lung cancer in vitro. Cell Mol Neurobiol. 2020;40(1):113–21.
Zheng Z, Chopp M, Chen J. Multifaceted roles of pericytes in central nervous system homeostasis and disease. J Cereb Blood Flow Metab. 2020;40(7):1381–401.
He BP, Wang JJ, Zhang X, Wu Y, Wang M, Bay B-H, et al. Differential reactions of microglia to brain metastasis of lung cancer. Mol Med. 2006;12(7):161–70.
Ransohoff RM, Cardona AE. The myeloid cells of the central nervous system parenchyma. Nature. 2010;468(7321):253–62.
Louie E, Chen X, Coomes A, Ji K, Tsirka S, Chen E. Neurotrophin-3 modulates breast cancer cells and the microenvironment to promote the growth of breast cancer brain metastasis. Oncogene. 2013;32(35):4064–77.
Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523(7560):337–41.
Hablitz LM, Plá V, Giannetto M, Vinitsky HS, Stæger FF, Metcalfe T, et al. Circadian control of brain glymphatic and lymphatic fluid flow. Nat Commun. 2020;11(1):1–11.
Song E, Mao T, Dong H, Boisserand LSB, Antila S, Bosenberg M, et al. VEGF-C-driven lymphatic drainage enables immunosurveillance of brain tumours. Nature. 2020;577(7792):689–94.
Brastianos PK, Carter SL, Santagata S, Cahill DP, Taylor-Weiner A, Jones RT, et al. Genomic characterization of brain metastases reveals branched evolution and potential therapeutic targets. Cancer Discov. 2015;5(11):1164–77.
Zhang Q, Zhang Y, Chen Y, Qian J, Zhang X, Yu K. A novel mTORC1/2 inhibitor (MTI-31) inhibits tumor growth, epithelial–mesenchymal transition, metastases, and improves antitumor immunity in preclinical models of lung cancer. Clin Cancer Res. 2019;25(12):3630–42.
Lee HY, Cha J, Kim SK, Park JH, Song KH, Kim P, et al. c-MYC drives breast cancer metastasis to the brain, but promotes synthetic lethality with TRAIL. Mol Cancer Res. 2019;17(2):544–54.
Eales KL, Hollinshead KE, Tennant DA. Hypoxia and metabolic adaptation of cancer cells. Oncogenesis. 2016;5(1): e190.
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, et al. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379(6560):88–91.
Lee KE, Simon MC. From stem cells to cancer stem cells: HIF takes the stage. Curr Opin Cell Biol. 2012;24(2):232–5.
Lee JW, Ko J, Ju C, Eltzschig HK. Hypoxia signaling in human diseases and therapeutic targets. Exp. Mol. Med. 2019:51(6);1–13.
Tirpe AA, Gulei D, Ciortea SM, Crivii C, Berindan-Neagoe I. Hypoxia: overview on hypoxiamediated mechanisms with a focus on the role of HIF genes. Int. J. Mol. Sci. 2019:20(24);6140.
Achrol AS, Rennert RC, Anders C, Soffietti R, Ahluwalia MS, Nayak L, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):1–26.
Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288–300.
Tsakonas G, De Petris L, Ekman S. Management of brain metastasized non-small cell lung cancer (NSCLC)–From local treatment to new systemic therapies. Cancer Treat Rev. 2017;54:122–31.
Cheng X, Hung MC. Breast cancer brain metastases. Cancer Metastasis Rev. 2007;26(3–4):635–43.
Lauko A, Rauf Y, Ahluwalia MS. Medical management of brain metastases. Neuro-Oncol Adv. 2020;2(1):015–015.
Suh JH. Efaproxiral: a novel radiation sensitiser. Expert Opin Investig Drugs. 2004;13(5):543–50.
Suh JH, Stea B, Nabid A, Kresl JJ, Fortin A, Mercier JP, et al. Phase III study of efaproxiral as an adjunct to whole-brain radiation therapy for brain metastases. J Clin Oncol. 2006;24(1):106–14.
Shaw E, Scott C, Suh J, Kadish S, Stea B, Hackman J, et al. RSR13 plus cranial radiation therapy in patients with brain metastases: comparison with the radiation therapy oncology group recursive partitioning analysis brain metastases database. J Clin Oncol. 2003;21(12):2364–71.
Russell SJ, Peng KW, Bell JC. Oncolytic virotherapy. Nat Biotechnol. 2012;30(7):658–70.
Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8.
Chesney J, Puzanov I, Collichio F, Singh P, Milhem MM, Glaspy J, et al. Randomized, open-label phase II study evaluating the efficacy and safety of talimogene laherparepvec in combination with ipilimumab versus ipilimumab alone in patients with advanced unresectable melanoma. J Clin Oncol. 2018;36(17):1658–67.
Ferguson MS, Lemoine NR, Wang Y. Systemic delivery of oncolytic viruses: hopes and hurdles. Adv Virol. 2012;2012: 805629.
Ikeda K, Ichikawa T, Wakimoto H, Silver JS, Deisboeck TS, Finkelstein D, et al. Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses. Nat Med. 1999;5(8):881–7.
Du W, Seah I, Bougazzoul O, Choi G, Meeth K, Bosenberg MW, et al. Stem cell-released oncolytic herpes simplex virus has therapeutic efficacy in brain metastatic melanomas. Proc Natl Acad Sci U S A. 2017;114(30):E6157-e6165.
Jiang H, Shin DH. Localized treatment with oncolytic adenovirus delta-24-RGDOX induces systemic immunity against. Dissem Subcutan Intracr Melan. 2019;25(22):6801–14.
Mansfield AS, Aubry MC, Moser JC, Harrington SM, Dronca RS, Park SS, et al. Temporal and spatial discordance of programmed cell death-ligand 1 expression and lymphocyte tumor infiltration between paired primary lesions and brain metastases in lung cancer. Ann Oncol. 2016;27(10):1953–8.
Kluger HM, Zito CR, Barr ML, Baine MK, Chiang VL, Sznol M, et al. Characterization of PD-L1 expression and associated T-cell infiltrates in metastatic melanoma samples from variable anatomic sites. Clin Cancer Res. 2015;21(13):3052–60.
Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016;17(7):976–83.
Long GV, Atkinson V, Lo S, Sandhu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19(5):672–81.
Jacobs JF, Idema AJ, Bol KF, Nierkens S, Grauer OM, Wesseling P, et al. Regulatory T cells and the PD-L1/PD-1 pathway mediate immune suppression in malignant human brain tumors. Neuro Oncol. 2009;11(4):394–402.
Spranger S, Spaapen RM, Zha Y, Williams J, Meng Y, Ha TT, et al. Up-regulation of PD-L1, IDO, and T(regs) in the melanoma tumor microenvironment is driven by CD8(+) T cells. Sci Transl Med. 2013;5(200):200116.
Steindl A, Alpar D, Heller G, Mair MJ, Gatterbauer B, Dieckmann K, et al. Tumor mutational burden and immune infiltrates in renal cell carcinoma and matched brain metastases. ESMO Open. 2021;6(2): 100057.
Zhao P, Zhang J, Wu A, Zhang M, Zhao Y, Tang Y, et al. Biomimetic codelivery overcomes osimertinib-resistant NSCLC and brain metastasis via macrophage-mediated innate immunity. J Control Release. 2021;329:1249–61.
Berghoff AS, Venur VA, Preusser M, Ahluwalia MS. Immune checkpoint inhibitors in brain metastases: from biology to treatment. Am Soc Clin Oncol Educ Book. 2016;36:e116–22.
Crowder RJ, Phommaly C, Tao Y, Hoog J, Luo J, Perou CM, et al. PIK3CA and PIK3CB inhibition produce synthetic lethality when combined with estrogen deprivation in estrogen receptor-positive breast cancer. Cancer Res. 2009;69(9):3955–62.
Miller TW, Hennessy BT, González-Angulo AM, Fox EM, Mills GB, Chen H, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120(7):2406–13.
Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12(4):342–54.
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68(15):6084–91.
Chen IC, Hsiao LP, Huang IW, Yu HC, Yeh LC, Lin CH, et al. Phosphatidylinositol-3 kinase inhibitors, buparlisib and alpelisib, sensitize estrogen receptor-positive breast cancer cells to tamoxifen. Sci Rep. 2017;7(1):9842.
Brosnan EM, Anders CK. Understanding patterns of brain metastasis in breast cancer and designing rational therapeutic strategies. Annals of translational medicine. 2018;6(9):163–163.
Owens T, Renno T, Taupin V, Krakowski M. Inflammatory cytokines in the brain: does the CNS shape immune responses? Immunol Today. 1994;15(12):566–71.
Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol. 2012;13(5):459–65.
Fitzgerald DP, Emerson DL, Qian Y, Anwar T, Liewehr DJ, Steinberg SM, et al. TPI-287, a new taxane family member, reduces the brain metastatic colonization of breast cancer cells. Mol Cancer Ther. 2012;11(9):1959–67.
Hu ZI, McArthur HL, Ho AY. The abscopal effect of radiation therapy: what is it and how can we use it in breast cancer? Curr Breast Cancer Rep. 2017;9(1):45–51.
Hsu PD, Lander ES, Zhang F. Development and applications of CRISPR-Cas9 for genome engineering. Cell. 2014;157(6):1262–78.
Wang T, Wei JJ, Sabatini DM, Lander ES. Genetic screens in human cells using the CRISPR-Cas9 system. Science. 2014;343(6166):80–4.
Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelsen TS, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343(6166):84–7.
Kühnöl J, Kühnöl C, Vordermark D. Radiotherapy of brain metastases from breast cancer: treatment results and prognostic factors. Oncol Lett. 2016;11(5):3223–7.
Chen Y, Jiang T, Zhang H, Gou X, Han C, Wang J, et al. LRRC31 inhibits DNA repair and sensitizes breast cancer brain metastasis to radiation therapy. Nat Cell Biol. 2020;22(10):1276–85.
Wang S, Liang K, Hu Q, Li P, Song J, Yang Y, et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Investig. 2017;127(12):4498–515.
Márquez-Ortiz RA, Contreras-Zárate MJ, Tesic V, Alvarez-Eraso KL, Kwak G, Littrell Z, et al. IL13Rα2 promotes proliferation and outgrowth of breast cancer brain metastases. Clin Cancer Res. 2021;27(22):6209–21.
Papageorgis P, Ozturk S, Lambert AW, Neophytou CM, Tzatsos A, Wong CK, et al. Targeting IL13Ralpha2 activates STAT6-TP63 pathway to suppress breast cancer lung metastasis. Breast Cancer Res. 2015;17(1):1–15.
Yoo B, Ross A, Pantazopoulos P, Medarova Z. MiRNA10b-directed nanotherapy effectively targets brain metastases from breast cancer. Sci Rep. 2021;11(1):1–7.
Masiero M, Nardo G, Indraccolo S, Favaro E. RNA interference: implications for cancer treatment. Mol Aspects Med. 2007;28(1):143–66.
Nam D-H, Jeon H-M, Kim S, Kim MH, Lee Y-J, Lee MS, et al. Activation of notch signaling in a xenograft model of brain metastasis. Clin Cancer Res. 2008;14(13):4059–66.
Wei Y, Sun Y, Wei J, Qiu X, Meng F, Storm G, et al. Selective transferrin coating as a facile strategy to fabricate BBB-permeable and targeted vesicles for potent RNAi therapy of brain metastatic breast cancer in vivo. J Control Release. 2021;337:521–9.
Acknowledgements
We acknowledge the Central University of Punjab for providing infrastructure and facilities. Council of Scientific and Industrial Research (CSIR), New Delhi, for Senior Research Fellowship (SRF) to Yogita Chhichholiya. and Junior Research Fellowship (JRF) to Ruthuparna Malayil for PhD. DST-FIST grant (SR/FST/LS-I/2017/49) to the Department of Human Genetics and Molecular Medicine, Central University of Punjab, is acknowledged with thanks.
Funding
Financial support to Yogita Chhichholiya (Award No- 09/1051(0038)/2019-EMR-1) and Ruthuparna Malayil (Award No- KL1216200313) from Council of Scientific and Industrial Research (CSIR) India is highly acknowledged.
Author information
Authors and Affiliations
Contributions
AM and YC conceptualized the idea of the review. YC, Rm and HV curated the data, and prepared the draft. AM critically revised and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chhichholiya, Y., Ruthuparna, M., Velagaleti, H. et al. Brain metastasis in breast cancer: focus on genes and signaling pathways involved, blood–brain barrier and treatment strategies. Clin Transl Oncol 25, 1218–1241 (2023). https://doi.org/10.1007/s12094-022-03050-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12094-022-03050-z