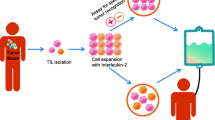
Abstract
The immune system plays a vital role in suppressing tumor cell progression. The tumor microenvironment augmented with significant levels of tumor-infiltrating lymphocytes has been widely investigated and it is suggested that tumor-infiltrating lymphocytes have shown a significant role in the prognosis of cancer patients. Compared to ordinary non-infiltrating lymphocytes, tumor-infiltrating lymphocytes (TILs) are a significant population of lymphocytes that infiltrate tumor tissue and have a higher level of specific immunological reactivity against tumor cells. They serve as an effective immunological defense against various malignancies. TILs are a diverse group of immune cells that are divided into immune subsets based on the pathological and physiological impact they have on the immune system. TILs mainly consist of B-cells, T-cells, or natural killer cells with diverse phenotypic and functional properties. TILs are known to be superior to other immune cells in that they can recognize a wide range of heterogeneous tumor antigens by producing many clones of T cell receptors (TCRs), outperforming treatments like TCR-T cell and CAR-T therapy. With the introduction of genetic engineering technologies, tumor-infiltrating lymphocytes (TILs) have become a ground-breaking therapeutic option for malignancies, but because of the hindrances opposed by the immune microenvironment and the mutation of antigens, the development of TILs as therapeutic has been hindered. By giving some insight into the many variables, such as the various barriers inhibiting its usage as a potential therapeutic agent, we have examined various aspects of TILs in this work.




Similar content being viewed by others
Data availability
The human single-cell omics dataset utilized for the study is freely available at https://www.immunesinglecell.org/ and the survival data can be accessed at http://timer.cistrome.org/
Abbreviations
- TIL:
-
Tumor-infiltrating lymphocyte
- TME:
-
Tumor microenvironment
- ECM:
-
Extracellular matrix
- ACT:
-
Adoptive cell therapy
- CCD2:
-
C-C chemokine receptor type 2
- NGFR:
-
Nerve growth factor receptor
- CXCR:
-
C-X-C chemokine receptor type
- BiAb:
-
Bispecific antibody
- CTL:
-
Cytotoxic T lymphocyte
- IL-8:
-
Interleukin-8
References
Badalamenti G, et al. Role of tumor-infiltrating lymphocytes in patients with solid tumors: can a drop dig a stone? Cell Immunol. 2019;343:103753.
Berghuis D, et al. Pro-inflammatory chemokine–chemokine receptor interactions within the Ewing sarcoma microenvironment determine CD8+ T-lymphocyte infiltration and affect tumour progression. J Pathol. 2011;223(3):347–57.
Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Investig. 2015;125(9):3384–91.
Foppen MHG, Donia M, Svane IM, Haanen J. Tumor-infiltrating lymphocytes for the treatment of metastatic cancer. Mol Oncol. 2015;9(10):1918–35.
Man Y-G, et al. Tumor-infiltrating immune cells promoting tumor invasion and metastasis: existing theories. J Cancer. 2013;4(1):84.
Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene. 2008;27(45):5904–12.
Mir MA, Agrewala JN. Influence of CD80 and CD86 co-stimulation in the modulation of the activation of antigen presenting cells. Curr Immunol Rev. 2007;3(3):160–9.
Weiss SA, et al. Immunologic heterogeneity of tumor-infiltrating lymphocyte composition in primary melanoma. Hum Pathol. 2016;57:116–25.
Mir MA, Agrewala JN. Signaling through CD80: an approach for treating lymphomas. Expert Opin Ther Targets. 2008;12(8):969–79.
Mahmoud SMA, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949–55.
Mehraj U, Mushtaq U, Mir MA, Saleem A, Macha MA, Lone MN, Hamid A, Zargar MA, Ahmad SM, Wani NA. Chemokines in triple-negative breast cancer heterogeneity: New challenges for clinical implications. Semin Cancer Biol. 2022;86(Pt 2):769–83.
Krynitz B, Rozell BL, Lyth J, Smedby KE, Lindelöf B. Cutaneous malignant melanoma in the Swedish organ transplantation cohort: a study of clinicopathological characteristics and mortality. J Am Acad Dermatol. 2015;73(1):106–13.
Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348(3):203–13.
Al-Shibli KI, Donnem T, Al-Saad S, Persson M, Bremnes RM, Busund L-T. Prognostic effect of epithelial and stromal lymphocyte infiltration in non–small cell lung cancer. Clin Cancer Res. 2008;14(16):5220–7.
Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J. Tumor infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51(1):90–5.
Mlecnik B, Bifulco C, Bindea G, Marliot F, Lugli A, Lee JJ, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Börger E. Multicenter international society for immunotherapy of cancer study of the consensus immunoscore for the prediction of survival and response to chemotherapy in stage III colon cancer. J Clin Oncol. 2020;38(31):3638.
Fukunaga A, et al. CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma. Pancreas. 2004;28(1):e26–31.
Kazemi MH, Sadri M, Najafi A, Rahimi A, Baghernejadan Z, Khorramdelazad H, Falak R. Tumor-infiltrating lymphocytes for treatment of solid tumors: It takes two to tango? Front Immunol. 2022;28(13):1018962.
Zito Marino F, et al. Are tumor-infiltrating lymphocytes protagonists or background actors in patient selection for cancer immunotherapy? Expert Opin Biol Ther. 2017;17(6):735–46.
Lin B, Du L, Li H, Zhu X, Cui L, Li X. Tumor-infiltrating lymphocytes: warriors fight against tumors powerfully. Biomed Pharmacother. 2020;132:110873.
Clark WH, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Can Res. 1969;29(3):705–27.
Clark WH Jr, et al. Model predicting survival in stage I melanoma based on tumor progression. JNCI J Nat Cancer Ins. 1989;81(24):1893–904.
Mehraj U, Alshehri B, Khan AA, Bhat AA, Bagga P, Wani NA, Mir MA. Expression pattern and prognostic significance of chemokines in breast cancer: an integrated bioinformatics analysis. Clin Breast Cancer. 2022;22(6):567–78.
Adams S, et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J Clin Oncol. 2014;32(27):2959.
Zacharakis N, et al. Immune recognition of somatic mutations leading to complete durable regression in metastatic breast cancer. Nat Med. 2018;24(6):724–30.
Creelan BC, et al. Tumor-infiltrating lymphocyte treatment for anti-PD-1-resistant metastatic lung cancer: a phase 1 trial. Nat Med. 2021;27(8):1410–8.
Tran E, et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science. 2014;344(6184):641–5.
Stevanović S, et al. Complete regression of metastatic cervical cancer after treatment with human papillomavirus–targeted tumor-infiltrating T cells. J Clin Oncol. 2015;33(14):1543.
Stevanović S, et al. A phase II study of tumor-infiltrating lymphocyte therapy for human papillomavirus–associated epithelial cancers TIL therapy for HPV-associated cancers. Clin Cancer Res. 2019;25(5):1486–93.
Jazaeri AA, Zsiros E, Amaria RN, Artz AS, Edwards RP, Wenham RM, Slomovitz BM, et al. Safety and efficacy of adoptive cell transfer using autologous tumor infiltrating lymphocytes (LN-145) for treatment of recurrent, metastatic, or persistent cervical carcinoma. J Clin Oncol. 2019;37(15):2538–2538.
Tran E, et al. T-cell transfer therapy targeting mutant KRAS in cancer. N Engl J Med. 2016;375(23):2255–62.
Zhao Y, et al. Tumor infiltrating lymphocyte (TIL) therapy for solid tumor treatment: progressions and challenges. Cancers. 2022;14(17):4160.
Oppermans N, Kueberuwa G, Hawkins RE, Bridgeman JS. Transgenic T-cell receptor immunotherapy for cancer: building on clinical success. Ther Adv Vaccines Immunother. 2020;8:2515135520933509.
Sarnaik A, Khushalani N, Chesney J, Kluger H, Curti B, Lewis K, Medina T, Thomas S, Pavlick A, Whitman E, Algarra S. P865 Safety & efficacy of lifileucel (LN-144) tumor infiltrating lymphocyte therapy in metastatic melanoma patients after progression on multiple therapies–independent review committee data update. J Immunother Cancer. 2020;8(Suppl 1).
Waldman AD, Fritz JM, Lenardo MJ. A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat Rev Immunol. 2020;20(11):651–68.
O'Rourke DM, et al. Abstract LB-053: Next-generation sequencing of T-cell receptor-beta gene rearrangements reveals dramatic expansion of T-cell clonotypes after CART-EGFRvIII therapy for glioblastoma. Cancer Res. 2017;77(13_Supplement):LB-053.
Müller WEG, Schröder HC, Wang X. Inorganic polyphosphates as storage for and generator of metabolic energy in the extracellular matrix. Chem Rev. 2019;119(24):12337–74.
Hung K. Hayashi R, Lafond-Walker A, Lowenstein C, Pardoll D, and Levitsky H, The central role of CD4+ T cells in the antitumor immune response. J Exp Med. 1998;188:2357–68.
Piersma SJ, et al. High number of intraepithelial CD8+ tumor-infiltrating lymphocytes is associated with the absence of lymph node metastases in patients with large early-stage cervical cancer. Can Res. 2007;67(1):354–61.
Pagès F, Galon J, Dieu-Nosjean M. C, Tartour E, Sautès-Fridman C, Fridman W-H, Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene. 2010;29:1093–102.
Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9.
Laghi L, et al. CD3+ cells at the invasive margin of deeply invading (pT3–T4) colorectal cancer and risk of post-surgical metastasis: a longitudinal study. Lancet Oncol. 2009;10(9):877–84.
Mir MA, Aisha S, Nisar S, Qayoom H, Mehraj U. Immuno-onco-metabolism and Therapeutic Resistance. In: Immuno-Oncology Crosstalk and Metabolism 2022 May 17 (pp. 45–89). Singapore: Springer Nature Singapore. https://link.springer.com/chapter/10.1007/978-981-16-6226-3_3
Qayoom H, Mehraj U, Aisha S, Sofi S, Mir MA. Integrating Immunotherapy with Chemotherapy: a New approach to drug repurposing. In: Drug Repurposing-Molecular Aspects and Therapeutic Applications. IntechOpen. 2022. https://www.intechopen.com/chapters/78716
Smyth MJ, Ngiow SF, Ribas A, Teng MWL. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13(3):143–58.
Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15(2):e58–68.
Denkert C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–13.
Kawazoe A, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017;20(3):407–15.
Dai C, et al. Concordance of immune checkpoints within tumor immune contexture and their prognostic significance in gastric cancer. Mol Oncol. 2016;10(10):1551–8.
Denkert C, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin oncol. 2015;33(9):983–91.
Denkert C, et al. Standardized evaluation of tumor-infiltrating lymphocytes in breast cancer: results of the ring studies of the international immuno-oncology biomarker working group. Mod Pathol. 2016;29(10):1155–64.
Yagi T, et al. PD-L1 expression, tumor-infiltrating lymphocytes, and clinical outcome in patients with surgically resected esophageal cancer. Ann Surg. 2019;269(3):471–8.
Edin S, et al. The prognostic importance of CD20+ B lymphocytes in colorectal cancer and the relation to other immune cell subsets. Sci Rep. 2019;9(1):1–9.
Cai X-Y, et al. Dendritic cell infiltration and prognosis of human hepatocellular carcinoma. J Cancer Res Clin Oncol. 2006;132(5):293–301.
Tanaka R, et al. Preoperative neutrophil-to-lymphocyte ratio predicts tumor-infiltrating CD8+ T cells in biliary tract cancer. Anticancer Res. 2020;40(5):2881–7.
Yoneda K, et al. Alteration in tumoural PD-L1 expression and stromal CD8-positive tumour-infiltrating lymphocytes after concurrent chemo-radiotherapy for non-small cell lung cancer. Br J Cancer. 2019;121(6):490–6.
Zhang Y-L, et al. Different subsets of tumor infiltrating lymphocytes correlate with NPC progression in different ways. Mol Cancer. 2010;9(1):1–11.
Thike AA, et al. Higher densities of tumour-infiltrating lymphocytes and CD4+ T cells predict recurrence and progression of ductal carcinoma in situ of the breast. Histopathology. 2020;76(6):852–64.
Baker K, Lachapelle J, Zlobec I, Bismar TA, Terracciano L, Foulkes WD. Prognostic significance of CD8+ T lymphocytes in breast cancer depends upon both oestrogen receptor status and histological grade. Histopathology. 2011;58(7):1107–16.
Klingen TA, Chen Y, Aas H, Wik E, Akslen LA. Tumor-associated macrophages are strongly related to vascular invasion, non-luminal subtypes, and interval breast cancer. Hum Pathol. 2017;69:72–80.
Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):1–15.
Huang XM, Zhang Y, Xu L, Wang M, Wang W. Clinical significance of tumor infiltrating lymphocytes in high-grade serous ovarian carcinoma. Zhonghua bing li xue za zhi = Chin J Pathol. 2019;48(8):610–4.
Nguyen N, et al. Tumor infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(7):1074–84.
Zhou J, et al. Clinicopathological implications of TIM3+ tumor-infiltrating lymphocytes and the miR-455-5p/Galectin-9 axis in skull base chordoma patients. Cancer Immunol Immunother. 2019;68(7):1157–69.
Xiao Y, et al. CD103+ T and dendritic cells indicate a favorable prognosis in oral cancer. J Dent Res. 2019;98(13):1480–7.
Antohe M, et al. Tumor infiltrating lymphocytes: The regulator of melanoma evolution. Oncol Lett. 2019;17(5):4155–61.
Mehraj U, Qayoom H, Mir MA. Prognostic significance and targeting tumor-associated macrophages in cancer: new insights and future perspectives. Breast Cancer. 2021;28(3):539–55.
Mehraj U, Qayoom H, Shafi S, Farhana P, Asdaq S, Mir MA. Cryptolepine targets TOP2A and inhibits tumor cell proliferation in breast cancer cells-an in vitro and in silico study. Anticancer Agents Med Chem (Formerly Current Medicinal Chemistry-Anti-Cancer Agents). 2022;22(17):3025–37.
Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother. 2020;69(1):3–14.
Mehraj U, Wani NA, Hamid A, Alkhanani M, Almilaibary A, Mir MA. Adapalene inhibits the growth of triple-negative breast cancer cells by S-phase arrest and potentiates the antitumor efficacy of GDC-0941. Front Pharmacol. 2022;13:958443.
Anderson NM, Simon MC. The tumor microenvironment. Curr Biol. 2020;30(16):R921–5.
Cohen IJ, Blasberg R. Impact of the tumor microenvironment on tumor-infiltrating lymphocytes: focus on breast cancer. Breast Cancer: Basic Clin Res. 2017;11:1178223417731565.
Mehraj U, Dar AH, Wani NA, Mir MA. Tumor microenvironment promotes breast cancer chemoresistance. Cancer Chemother Pharmacol. 2021;87(2):147–58.
McHugh MI, et al. Immunosuppression with polyunsaturated fatty acids in renal transplantation. Transplantation. 1977;24(4):263–7.
Carrillo Pérez C, CaviaCamarero MDM, Alonso de la Torre S. Role of oleic acid in immune system; mechanism of action; a review. Nutri Hosp. 2012;27 (4):978–990.
Richieri GV, Mescher MF, Kleinfeld AM. Short term exposure to cis unsaturated free fatty acids inhibits degranulation of cytotoxic T lymphocytes. J Immunol. 1990;144(2):671–7.
Mehraj U, Ganai RA, Macha MA, Hamid A, Zargar MA, Bhat AA, Nasser MW, Haris M, Batra SK, Alshehri B, Al-Baradie RS. The tumor microenvironment as driver of stemness and therapeutic resistance in breast cancer: New challenges and therapeutic opportunities. Cellular Oncology. 2021 Dec 1:1-21.
Mehraj U, Aisha S, Sofi S, Mir MA. Expression pattern and prognostic significance of baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5) in breast cancer: a comprehensive analysis. Adv Cancer Biol-Metastasis. 2022;4:100037.
Cheeseman KH. Mechanisms and effects of lipid peroxidation. Mol Aspects Med. 1993;14(3):191–7.
DeBerardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr Opin Genet Dev. 2008;18(1):54–61.
O’Flanagan CH, Bowers LW, Hursting SD. A weighty problem: metabolic perturbations and the obesity-cancer link. Horm Mol Biol Clin Invest. 2015;23(2):47–57.
Nieman KM, et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat Med. 2011;17(11):1498–503.
Zaidi N, Lupien L, Kuemmerle NB, Kinlaw WB, Swinnen JV, Smans K. Lipogenesis and lipolysis: the pathways exploited by the cancer cells to acquire fatty acids. Prog Lipid Res. 2013;52(4):585–9.
Kakinuma T, Hwang ST. Chemokines, chemokine receptors, and cancer metastasis. J Leukoc Biol. 2006;79(4):639–51.
Nishi K, et al. Inhibition of fatty acid synthesis induces apoptosis of human pancreatic cancer cells. Anticancer Res. 2016;36(9):4655–60.
Pearce EL, et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature. 2009;460(7251):103–7.
Sofi S, et al. Cyclin-dependent kinases in breast cancer: expression pattern and therapeutic implications. Med Oncol. 2022;39(6):1–16.
Oberholtzer N, Quinn KM, Chakraborty P, Mehrotra S. New developments in T cell immunometabolism and implications for cancer immunotherapy. Cells. 2022;11(4):708.
Scharping NE, et al. The tumor microenvironment represses T cell mitochondrial biogenesis to drive intratumoral T cell metabolic insufficiency and dysfunction. Immunity. 2016;45(2):374–88.
Pereira-Nunes A, Afonso J, Granja S, Baltazar F. Lactate and lactate transporters as key players in the maintenance of the Warburg effect. Tumor Microenvironment: The Main Driver of Metabolic Adaptation. 2020:51-74.
Manthey JA, Grohmann K, Montanari A, Ash K, Manthey CL. Polymethoxylated flavones derived from citrus suppress tumor necrosis factor-α expression by human monocytes. J Nat Prod. 1999;62(3):441–4.
Brand A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 2016;24(5):657–71.
de la Cruz-López KG, Castro-Muñoz LJ, Reyes-Hernández DO, García-Carrancá A, Manzo-Merino J. Lactate in the regulation of tumor microenvironment and therapeutic approaches. Front Oncol. 2019;9:1143.
Parks SK, Chiche J, Pouysségur J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat Rev Cancer. 2013;13(9):611–23.
Sofi S, et al. Targeting cyclin-dependent kinase 1 (CDK1) in cancer: molecular docking and dynamic simulations of potential CDK1 inhibitors. Med Oncol. 2022;39(9):1–15.
Peppicelli S, et al. Metformin is also effective on lactic acidosis-exposed melanoma cells switched to oxidative phosphorylation. Cell Cycle. 2016;15(14):1908–18.
Talasila KM, et al. The angiogenic switch leads to a metabolic shift in human glioblastoma. Neuro Oncol. 2017;19(3):383–93.
Qayoom H, Wani NA, Alshehri B, Mir MA. An insight into the cancer stem cell survival pathways involved in chemoresistance in triple-negative breast cancer. Future Oncol. 2021;17(31):4185–206.
Guillaumond F, et al. Strengthened glycolysis under hypoxia supports tumor symbiosis and hexosamine biosynthesis in pancreatic adenocarcinoma. Proc Natl Acad Sci. 2013;110(10):3919–24.
Cohen R, Neuzillet C, Tijeras-Raballand A, Faivre S, de Gramont A, Raymond E. Targeting cancer cell metabolism in pancreatic adenocarcinoma. Oncotarget. 2015;6(19):16832.
Colegio OR, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513(7519):559–63.
Kai F, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell. 2019;49(3):332–46.
Jarkovska K, Dvorankova B, Halada P, Kodet O, Szabo P, Gadher SJ, Motlik J, Kovarova H, Smetana K Jr. Revelation of fibroblast protein commonalities and differences and their possible roles in wound healing and tumourigenesis using co-culture models of cells. Biology of the Cell. 2014;106(7):203–18.
Mali AV, Joshi AA, Hegde MV, Kadam SS. Enterolactone suppresses proliferation, migration and metastasis of MDA-MB-231 breast cancer cells through inhibition of uPA induced plasmin activation and MMPs-mediated ECM remodeling. Asian Pac J Cancer Prev: APJCP. 2017;18(4):905.
Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Can Res. 2010;70(15):6171–80.
Wong NS, et al. Phase I/II trial of metronomic chemotherapy with daily dalteparin and cyclophosphamide, twice-weekly methotrexate, and daily prednisone as therapy for metastatic breast cancer using vascular endothelial growth factor and soluble vascular endothelial growth factor receptor levels as markers of response. J Clin Oncol. 2010;28(5):723–30.
Klinger M, Benjamin J, Kischel R, Stienen S, Zugmaier G. Harnessing T cells to fight cancer with BiTE® antibody constructs–past developments and future directions. Immunol Rev. 2016;270(1):193–208.
Kobold S, et al. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J Nat Cancer Inst. 2015;107(1):dju364.
Sapoznik S, et al. CXCR1 as a novel target for directing reactive T cells toward melanoma: implications for adoptive cell transfer immunotherapy. Cancer Immunol Immunother. 2012;61(10):1833–47.
Mir MA, Agrewala JN. Signaling through CD80: an approach for treating lymphomas. Expert Opin Ther Targets. 2008;12(8):969–79.
Rapp M, et al. CC chemokine receptor type-4 transduction of T cells enhances interaction with dendritic cells, tumor infiltration and therapeutic efficacy of adoptive T cell transfer. Oncoimmunology. 2016;5(3): e1105428.
Moon EK, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17(14):4719–30.
Zhang J, Endres S, Kobold S. Enhancing tumor T cell infiltration to enable cancer immunotherapy. Immunotherapy. 2019;11(3):201–13.
Fields GB. The rebirth of matrix metalloproteinase inhibitors: moving beyond the dogma. Cells. 2019;8(9):984.
Dezube BJ, Krown SE, Lee JY, Bauer KS, Aboulafia DM. Randomized phase II trial of matrix metalloproteinase inhibitor COL-3 in AIDS-related Kaposi’s sarcoma: an AIDS Malignancy Consortium Study. J Clin Oncol. 2006;24(9):1389–94.
Jin M-Z, Jin W-L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5(1):1–16.
Zhong S, Jeong J-H, Chen Z, Chen Z, Luo J-L. Targeting tumor microenvironment by small-molecule inhibitors. Trans Oncol. 2020;13(1):57–69.
Ho P-C, Kaech SM. Reenergizing T cell anti-tumor immunity by harnessing immunometabolic checkpoints and machineries. Curr Opin Immunol. 2017;46:38–44.
Beloribi-Djefaflia S, Vasseur S, Guillaumond F. Lipid metabolic reprogramming in cancer cells. Oncogenesis. 2016;5(1):e189–e189.
Harisi R, Jeney A. Extracellular matrix as target for antitumor therapy. Onco Targets Ther. 2015;8:1387.
Spranger S, et al. Density of immunogenic antigens does not explain the presence or absence of the T-cell–inflamed tumor microenvironment in melanoma. Proc Natl Acad Sci. 2016;113(48):E7759–68.
Safonov A, et al. Immune gene expression is associated with genomic aberrations in breast cancer. Can Res. 2017;77(12):3317–24.
Creelan B, Wang C, Teer J, Toloza E, Mullinax J, Yao J, Koomen J, Kim S, Chiappori A, Saller J, Montoya L. Abstract CT056: Durable complete responses to adoptive cell transfer using tumor infiltrating lymphocytes (TIL) in non-small cell lung cancer (NSCLC): A phase I trial. Cancer research. 2020;80(16_Supplement):CT056-.
Wang C, Li M, Wei R, Wu J. Adoptive transfer of TILs plus anti-PD1 therapy: an alternative combination therapy for treating metastatic osteosarcoma. Journal of Bone Oncology. 2020;25:100332.
Acknowledgements
The authors are thankful to the Jammu and Kashmir Science Technology & Innovation council DST Govt of J&K for financial support to this study.
Funding
This work was funded by the Jammu Kashmir Science Technology & Innovation council Department of Science and Technology (JKDST) India with Grant No. JKST&IC/SRE/885–87 to Dr. Manzoor Ahmad Mir.
Author information
Authors and Affiliations
Contributions
MAM designed and supervised the work. HQ wrote the manuscript, designed the figures, and edited the manuscript. MAM, HQ, and SS critically revised and edited the manuscript. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qayoom, H., Sofi, S. & Mir, M.A. Targeting tumor microenvironment using tumor-infiltrating lymphocytes as therapeutics against tumorigenesis. Immunol Res 71, 588–599 (2023). https://doi.org/10.1007/s12026-023-09376-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-023-09376-2