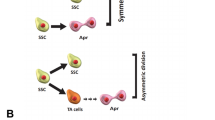
Abstract
Background
Spermatogonia Stem Cells (SSCs) are potential candidates for reprogramming and regeneration. Recent studies have revealed that differentiated cells can be reverted to pluripotent by overexpressing a set of pluripotent transcription factors. OCT4 (encoded by pou5f1), a POU transcription factor family member, is essential to the potential that controls pluripotency, and it is widely expressed in pluripotent stem cells, although it decreased or suppressed after differentiation.
Methods
In this investigated research, we examined the OCT4 expression during the differentiation of SSCs into neurons (involving four stages in the following order: SSCs in vivo and in-vitro, embryonic Stem Cell-like (ES-like), Embryonic Bodies (EBs), and finally Neurons) by Immunocytochemistry (ICC), Immunohistochemistry (IMH), and Fluidigm Real-Time polymerase chain reaction. In addition, we use some databases like STRING to predict protein–protein interaction and enrichment analysis.
Results
We evaluated the expression of OCT4 in this process, and we observed that it is expressed in SSCs, ES-like, and EBs during the differentiation of spermatogonia stem cells into adult neurons. We show that by adding RA to EBs, the expression of OCT4 is reduced and is not expressed in the neuron cells. We observed that the expression of OCT4 is linked and interacts with the differentiation of spermatogonia stem cells into neuron cells, and it has been shown to be biologically functional, like stem cell maintenance and somatic cell reprogramming.
Conclusion
Our findings can help us better understand the process of differentiation of spermatogonia stem cells into neurons, and it can be effective in finding new and more efficient treatments for neurogenesis and repair of neurons.
Graphical Abstract
We examined the OCT4 expression during the differentiation of SSCs into neurons (involving four stages in the following order: SSCs in vivo and in-vitro, embryonic Stem Cell-like (ES-like), Embryonic Bodies (EBs), and finally Neurons) by Immunocytochemistry (ICC), Immunohistochemistry (IMH), and Fluidigm Real-Time polymerase chain reaction. In addition, we use some databases like STRING to predict protein–protein interaction and enrichment analysis. We evaluated the expression of OCT4 in this process, and we observed that it is expressed in SSCs, ES-like, and EBs during the differentiation of spermatogonia stem cells into adult neurons. We show that by adding RA to EBs, the expression of OCT4 is reduced and is not expressed in the neuron cells. We observed that the expression of OCT4 is linked and interacts with the differentiation of spermatogonia stem cells into neuron cells, and it has been shown to be biologically functional, like stem cell maintenance and somatic cell reprogramming.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are available from the corresponding author on reasonable request.
Abbreviations
- SSCs:
-
Spermatogonia Stem Cells
- ICC:
-
Immunocytochemistry
- IHC:
-
Immunohistochemistry
- ES-Like:
-
Embryonic Stem Cell-like
- EBs:
-
Embryoid bodies
- iPSCs:
-
Induced pluripotent stem cells
- PPI:
-
Protein–protein interactions
- RA:
-
Retinoic acid
References
Kahroba, H., et al. (2021). The role of Nrf2 in neural stem/progenitors cells: From maintaining stemness and self-renewal to promoting differentiation capability and facilitating therapeutic application in neurodegenerative disease. Ageing research reviews, 65, 101211.
Maher, P. (2019). The potential of flavonoids for the treatment of neurodegenerative diseases. International journal of molecular sciences, 20(12), 3056.
Bojnordi, M. N., et al. (2017). Differentiation of spermatogonia stem cells into functional mature neurons characterized with differential gene expression. Molecular neurobiology, 54(7), 5676–5682.
Strnadel, J., et al. (2018). Survival of syngeneic and allogeneic iPSC-derived neural precursors after spinal grafting in minipigs. Science Translational Medicine, 10(440), eaam6651. https://doi.org/10.1126/scitranslmed.aam6651
Little, D., et al. (2019). Using stem cell–derived neurons in drug screening for neurological diseases. Neurobiology of aging, 78, 130–141.
Hall, B. K. (2018). Germ layers, the neural crest and emergent organization in development and evolution. Genesis, 56(6-7), e23103. https://doi.org/10.1002/dvg.23103
Shafa, M., et al. (2018). Human-induced pluripotent stem cells manufactured using a current good manufacturing practice-compliant process differentiate into clinically relevant cells from three germ layers. Frontiers in medicine, 5, 69.
Katayama, S., et al. (2018). HDAC8 regulates neural differentiation through embryoid body formation in P19 cells. Biochemical and biophysical research communications, 498(1), 45–51.
Yang, J., et al. (2017). Analysis of retinoic acid-induced neural differentiation of mouse embryonic stem cells in two and three-dimensional embryoid bodies. JoVE (Journal of Visualized Experiments), 122, e55621.
Sinha, N., et al. (2021). Roles of Stra8 and Tcerg1l in retinoic acid induced spermatogonial differentiation in mouse. Biology of Reproduction, 105(2), 503–518.
Zhang, C., et al. (2017). Biological significance of the suppression of oxidative phosphorylation in induced pluripotent stem cells. Cell reports, 21(8), 2058–2065.
Li, Y.-Q. (2017). Networks of transcription factors for OCT expression in mice. DNA and cell biology, 36(9), 725–736.
Echigoya, K., et al. (2020). Nucleosome binding by the pioneer transcription factor OCT4. Scientific reports, 10(1), 1–11.
Zalc, A., et al. (2021). Reactivation of the pluripotency program precedes formation of the cranial neural crest. Science, 371(6529), eabb4776. https://doi.org/10.1126/science.abb4776
Azizi, H., Asgari, B., & Skutella, T. (2019). Pluripotency potential of embryonic stem cell-like cells derived from mouse testis. Cell Journal (Yakhteh), 21(3), 281.
Conrad, S., et al. (2016). Expression of genes related to germ cell lineage and pluripotency in single cells and colonies of human adult germ stem cells. Stem Cells Int, 2016, 8582526. https://doi.org/10.1155/2016/8582526
Azizi, H., et al. (2016). Derivation of pluripotent cells from mouse SSCs seems to be age dependent. Stem Cells Int, 2016, 8216312. https://doi.org/10.1155/2016/8216312
Azizi, H., Hamidabadi, H. G., & Skutella, T. (2019). Differential proliferation effects after short-term cultivation of mouse spermatogonial stem cells on different feeder layers. Cell Journal (Yakhteh), 21(2), 186.
Mizrak, S. C., et al. (2010). Embryonic stem cell-like cells derived from adult human testis. Human Reproduction, 25(1), 158–167.
Kaushik, A., & Bhartiya, D. (2018). Pluripotent very small embryonic-like stem cells in adult testes–an alternate premise to explain testicular germ cell tumors. Stem cell reviews and reports, 14(6), 793–800.
Yamashiro, C., et al. (2018). Generation of human oogonia from induced pluripotent stem cells in vitro. Science, 362(6412), 356–360.
Akamatsu, W., et al. (2009). Suppression of OCT4 by germ cell nuclear factor restricts pluripotency and promotes neural stem cell development in the early neural lineage. Journal of Neuroscience, 29(7), 2113–2124.
Johnstone, V. P., et al. (2015). Experimental traumatic brain injury results in long-term recovery of functional responsiveness in sensory cortex but persisting structural changes and sensorimotor, cognitive, and emotional deficits. Journal of neurotrauma, 32(17), 1333–1346.
Mattson, M. P. (2000). Apoptosis in neurodegenerative disorders. Nature reviews Molecular cell biology, 1(2), 120–130.
Bhowmick, S., et al. (2018). Neurodegeneration and sensorimotor deficits in the mouse model of traumatic brain injury. Brain sciences, 8(1), 11.
Yang, H., et al. (2019). Sonic Hedgehog Effectively Improves OCT4-Mediated Reprogramming of Astrocytes into Neural Stem Cells. Molecular Therapy, 27(8), 1467–1482.
Carreon, L. Y., & Dimar, J. R. (2011). Early versus late stabilization of spine injuries: A systematic review. Spine, 36(11), E727–E733.
Vismara, I., et al. (2017). Current options for cell therapy in spinal cord injury. Trends in molecular medicine, 23(9), 831–849.
Baptiste, D. C., & Fehlings, M. G. (2007). Update on the treatment of spinal cord injury. Progress in brain research, 161, 217–233.
Lepore, A., et al. (2006). Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience, 142(1), 287–304.
Martínez-Serrano, A., & Björklund, A. (1997). Immortalized neural progenitor cells for CNS gene transfer and repair. Trends in neurosciences, 20(11), 530–538.
Pessina, A., & Gribaldo, L. (2006). The key role of adult stem cells: Therapeutic perspectives. Current Medical Research and Opinion, 22(11), 2287–2300.
Gruen, L., & Grabel, L. (2006). Concise review: Scientific and ethical roadblocks to human embryonic stem cell therapy. Stem cells, 24(10), 2162–2169.
Swijnenburg, R.-J., et al. (2008). Immunosuppressive therapy mitigates immunological rejection of human embryonic stem cell xenografts. Proceedings of the National Academy of Sciences, 105(35), 12991–12996.
Gomes, K. M., Costa, I. C., Santos, J. F., Dourado, P. M., Forni, M. F., & Ferreira, J. C. (2017). Induced pluripotent stem cells reprogramming: Epigenetics and applications in the regenerative medicine. Rev Assoc Med Bras (1992), 63(2), 180–189. https://doi.org/10.1590/1806-9282.63.02.180
Mertens, J., et al. (2016). Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nature Reviews Neuroscience, 17(7), 424–437.
Kim, S. M., et al. (2014). Direct conversion of mouse fibroblasts into induced neural stem cells. Nature protocols, 9(4), 871–881.
Han, D. W., et al. (2012). Direct reprogramming of fibroblasts into neural stem cells by defined factors. Cell Stem Cell, 10(4), 465–472.
Lujan, E., et al. (2012). Direct conversion of mouse fibroblasts to self-renewing, tripotent neural precursor cells. Proceedings of the National Academy of Sciences, 109(7), 2527–2532.
Zhang, M., et al. (2016). Pharmacological reprogramming of fibroblasts into neural stem cells by signaling-directed transcriptional activation. Cell Stem Cell, 18(5), 653–667.
Zhu, S., Wang, H., & Ding, S. (2015). Reprogramming fibroblasts toward cardiomyocytes, neural stem cells and hepatocytes by cell activation and signaling-directed lineage conversion. Nature protocols, 10(7), 959–973.
Yang, H., et al. (2011). ErbB2 activation contributes to de-differentiation of astrocytes into radial glial cells following induction of scratch-insulted astrocyte conditioned medium. Neurochemistry international, 59(7), 1010–1018.
Feng, G.-D., et al. (2014). Fibroblast growth factor 4 is required but not sufficient for the astrocyte dedifferentiation. Molecular neurobiology, 50, 997–1012.
Takuma, K., Baba, A., & Matsuda, T. (2004). Astrocyte apoptosis: Implications for neuroprotection. Progress in neurobiology, 72(2), 111–127.
Masoudi, M., Azizi, H., Sojoudi, K., Yazdani, M., & Gholami, D. (2022). Comparison of POU5F1 gene expression and protein localization in two differentiated and undifferentiated spermatogonial stem cells. Biol Futur, 73(4), 503–512. https://doi.org/10.1007/s42977-022-00149-w
Niazi Tabar, A., et al. (2022). Testicular Localization and Potential Function of Vimentin Positive Cells during Spermatogonial Differentiation Stages. Animals, 12(3), 268.
Amirian, M., et al. (2022). VASA protein and gene expression analysis of human non-obstructive azoospermia and normal by immunohistochemistry, immunocytochemistry, and bioinformatics analysis. Scientific Reports, 12(1), 17259.
Conrad, S., Azizi, H., Hatami, M., Kubista, M., Bonin, M., Hennenlotter, J., Renninger, M., & Skutella, T. (2014). Differential gene expression profiling of enriched human spermatogonia after short- and long-term culture. Biomed Res Int, 2014, 138350. https://doi.org/10.1155/2014/138350
Hashemi Karoii, D., & Azizi, H. (2022). A review of protein-protein interaction and signaling pathway of Vimentin in cell regulation, morphology and cell differentiation in normal cells. J Recept Signal Transduct Res, 42(5), 512–520. https://doi.org/10.1080/10799893.2022.2047199
Karoii, D.H., Azizi H., & Amirian M. (2022) Signaling Pathways and Protein–Protein Interaction of Vimentin in Invasive and Migration Cells: A Review. Cellular Reprogramming.
Azizi, H., Skutella, T., & Shahverdi, A. (2017). Generation of mouse spermatogonial stem-cell-colonies in a non-adherent culture. Cell Journal (Yakhteh), 19(2), 238.
Azizi, H., Hashemi Karoii, D., & Skutella, T. (2022). Whole Exome Sequencing and In Silico Analysis of Human Sertoli in Patients with Non-Obstructive Azoospermia. Int J Mol Sci., 23(20), 12570. https://doi.org/10.3390/ijms232012570
Hashemi Karoii, D., Azizi, H., & Skutella, T. (2022). Microarray and in silico analysis of DNA repair genes between human testis of patients with nonobstructive azoospermia and normal cells. Cell Biochem Funct., 40(8), 865–879. https://doi.org/10.1002/cbf.3747
Szklarczyk, D., et al. (2019). STRING v11: Protein–protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic acids research, 47(D1), D607–D613.
Doncheva, N. T., et al. (2018). Cytoscape StringApp: Network analysis and visualization of proteomics data. Journal of proteome research, 18(2), 623–632.
Zhang, S., et al. (2019). OCT4 and PAX6 determine the dual function of SOX2 in human ESCs as a key pluripotent or neural factor. Stem cell research & therapy, 10(1), 1–14.
Ding, S., et al. (2021). CHD8 safeguards early neuroectoderm differentiation in human ESCs and protects from apoptosis during neurogenesis. Cell death & disease, 12(11), 1–15.
Lei, I., et al. (2020). SWI/SNF component BAF250a coordinates OCT4 and WNT signaling pathway to control cardiac lineage differentiation. Frontiers in cell and developmental biology, 7, 358.
Masoudi, M., Azizi, H., Sojoudi, K., Yazdani, M., & Gholami, D. (2022). Comparison of POU5F1 gene expression and protein localization in two differentiated and undifferentiated spermatogonial stem cells. Biol Futur., 73(4), 503–512. https://doi.org/10.1007/s42977-022-00149-w
Niknejad, P., Azizi, H., & Sojoudi, K. (2021). POU5F1 Protein and Gene Expression Analysis in Neonate and Adult Mouse Testicular Germ Cells by Immunohistochemistry and Immunocytochemistry. Cell Reprogram., 23(6), 349–358. https://doi.org/10.1089/cell.2021.0108
Nogales, F. F., et al. (2018). Germ cell tumour growth patterns originating from clear cell carcinomas of the ovary and endometrium: A comparative immunohistochemical study favouring their origin from somatic stem cells. Histopathology, 72(4), 634–647.
Tong, M.-H., et al. (2011). Expression of Mirlet7 family microRNAs in response to retinoic acid-induced spermatogonial differentiation in mice. Biology of Reproduction, 85(1), 189–197.
Villodre, E. S., et al. (2019). Silencing of the transcription factors OCT4, Sox2, Klf4, c-Myc or Nanog has different effect on teratoma growth. Biochemical and biophysical research communications, 517(2), 324–329.
Busada, J. T., et al. (2015). Retinoic acid regulates Kit translation during spermatogonial differentiation in the mouse. Developmental biology, 397(1), 140–149.
Simmet, K., et al. (2018). OCT4/POU5F1 is required for NANOG expression in bovine blastocysts. Proceedings of the National Academy of Sciences, 115(11), 2770–2775.
Kirsanov, O., et al. (2022). Modeling mammalian spermatogonial differentiation and meiotic initiation in vitro. Development, 149(22), dev200713. https://doi.org/10.1242/dev.200713
Wang, X., et al. (2015). Isolation and culture of pig spermatogonial stem cells and their in vitro differentiation into neuron-like cells and adipocytes. International Journal of Molecular Sciences, 16(11), 26333–26346.
Gilmozzi, V., et al. (2021). Generation of hiPSC-derived functional dopaminergic neurons in alginate-based 3D culture. Frontiers in Cell and Developmental Biology, 9, 708389.
Acknowledgements
This research was supported by Amol University of Special Modern Technologies (AUSMT) and the University of Heidelberg, Institute for Anatomy and Cell Biology III, Department of Neuroanatomy, Germany.
Funding
This research was funded by Centre for International Scientific Studies and Collaboration (CISSC), Ministry of Science, Research and Technology, and a BMBF grant (01DS13003). This research has been done with the financial support of the Centre for International Scientific Studies and Collaboration (CISSC) of the Ministry of Science, Research and Technology as well as the Iranian National Science Foundation (INSF) under project No.4006239, and a memorandum of understanding (MOU) agreement between the Institute for Anatomy and Cell Biology at the University of Heidelberg and Amol University of Special Modern Technology.
Author information
Authors and Affiliations
Contributions
H.A: Carried out and designed the experiment and edited the manuscript, and D.H.K: Assembly of data, do the experiment, data analysis, design PPI network, and enrichment analysis, wrote and edited the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All animal use was approved by University of Heidelberg and Amol Research Ethics Office and experiments were conducted in accordance with the Iranian Council of Animal Care Policies.
Consent to Participat
Not applicable.
Consent for Publication
We consent to publication.
Conflict of Interests
The authors declare that there is no conflict of interest regarding the publication of this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashemi Karoii, D., Azizi, H. OCT4 protein and gene expression analysis in the differentiation of spermatogonia stem cells into neurons by immunohistochemistry, immunocytochemistry, and bioinformatics analysis. Stem Cell Rev and Rep 19, 1828–1844 (2023). https://doi.org/10.1007/s12015-023-10548-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-023-10548-8