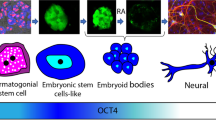
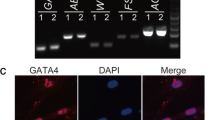
Abstract
Purpose
The signaling pathway of spermatogenesis may be identified by analyzing and contrasting the similarities and differences in the expression and regulation of important pluripotent stem cell genes during spermatogenesis.
Methods
In this study, after isolating spermatogonial, embryonic stem cells and embryonic stem–like cells (ES-like) were prepared. The expression of alkaline phosphatase is then measured, and then it was discovered how genes directly connected to alkaline phosphatase interact by investigating their protein-protein interaction network. In the following, Fluidigm PCR and immunocytochemistry are utilized in spermatogonial, embryonic, and ES-like stem cells to evaluate the expression of Sox2, Oct4, and Nanog genes.
Results
Alkaline phosphatase is expressed positively in spermatogonial stem cells and pluripotent stem cells but not in Sertoli cells or fibroblasts, indicating that this enzyme is exclusive to these cell types. Sox2, Oct4, and Nanog have a positive and significant expression (P<0.05) in spermatogonial, embryonic stem cells, and ES-like cells, and a decreased expression is observed in differentiated and Sertoli cells.
Conclusion
Alkaline phosphatase and the genes Sox2, Oct4, and Nanog, which have a critical role in regulating the spermatogenesis process, have a regulatory role on each other’s activity and have a specific expression pattern in pluripotent and spermatogonial stem cells.
Lay Summary
The expression of Sox2, Oct4, and Nanog genes are very vital in regulating the expression of other genes and factors involved in the control and regulation of spermatogenesis. Therefore, examining their expression level and also how they are related can be helpful in the finding causes of infertility diseases. Also, investigating the sources of pluripotent stem cells like ES-like cells can be very important in relation to the emergence of new cell therapy methods.
Graphical Abstract







Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
Abbreviations
- Oct4:
-
octamer-binding transcription factor 4
- Sox2:
-
Sry-related box-2
- IMH:
-
immunohistochemistry
- ICC:
-
immunocytochemistry
- SSCs:
-
spermatogonial stem cells
- PTMs:
-
peritubular myoid cells
- SCs:
-
Sertoli cells
- PGCs:
-
primordial germ cells
References
Štefková K, Procházková J, Pacherník J. Alkaline phosphatase in stem cells, vol. 2015. Stem cells international; 2015.
McComb RB, Bowers GN Jr, Posen S. Alkaline phosphatase. Springer Science & Business Media; 2013.
Sato M, et al. Tissue-nonspecific alkaline phosphatase, a possible mediator of cell maturation: towards a new paradigm. Cells. 2021;10(12):3338.
McGowan SL, et al. Stem cell markers in the human posterior limbus and corneal endothelium of unwounded and wounded corneas. Mol Vis. 2007;13(223-27):1984–2000.
Kermer V, et al. Knockdown of tissue nonspecific alkaline phosphatase impairs neural stem cell proliferation and differentiation. Neurosci Lett. 2010;485(3):208–11.
Goldberg RF, et al. Intestinal alkaline phosphatase is a gut mucosal defense factor maintained by enteral nutrition. Proc Natl Acad Sci U S A. 2008;105(9):3551–6.
Blomberg LA, Schreier LL, Talbot NC. Expression analysis of pluripotency factors in the undifferentiated porcine inner cell mass and epiblast during in vitro culture. Molecular Reprod and Dev: Incorporating Gamete Res. 2008;75(3):450–63.
Yamaguchi S, et al. Nanog expression in mouse germ cell development. Gene Expr Patterns. 2005;5(5):639–46.
Zhong C, et al. Pou5f1 and nanog are reliable germ cell-specific genes in gonad of a protogynous hermaphroditic fish, orange-spotted grouper (Epinephelus coioides). Genes. 2021;13(1):79.
Zheng Y, et al. Ectopic POU5F1 in the male germ lineage disrupts differentiation and spermatogenesis in mice. Reproduction (Cambridge, England). 2016;152(4):363.
Niknejad P, Azizi H, Sojoudi K. POU5F1 protein and gene expression analysis in neonate and adult mouse testicular germ cells by immunohistochemistry and immunocytochemistry. Cellular Reprog. 2021;23(6):349–58.
Liu R, et al. Medaka Oct4 is essential for pluripotency in blastula formation and ES cell derivation. Stem Cell Rev Rep. 2015;11(1):11–23.
Malik V, et al. Pluripotency reprogramming by competent and incompetent POU factors uncovers temporal dependency for Oct4 and Sox2. Nat comm. 2019;10(1):1–16.
Oatley JM. Recent advances for spermatogonial stem cell transplantation in livestock. Reproduction, fertility and Dev. 2018;30(1):44–9.
Kurimoto K, Saitou M. Germ cell reprogramming. Curr Top Dev Biol. 2019;135:91–125.
Karagiannis P, et al. Induced pluripotent stem cells and their use in human models of disease and development. Phys rev. 2019;99(1):79–114.
Driessens G, Blanpain C. Long live Sox2: Sox2 lasts a lifetime. Cell stem cell. 2011;9(4):283–4.
Xiao W, et al. SOX2 promotes brain metastasis of breast cancer by upregulating the expression of FSCN1 and HBEGF. Mol Ther - Oncolytics. 2020;17:118–29.
Azizi H, Niazi Tabar A, Skutella T. Successful transplantation of spermatogonial stem cells into the seminiferous tubules of busulfan-treated mice. Reprod health. 2021;18(1):1–9.
Masoudi M, et al. Comparison of POU5F1 gene expression and protein localization in two differentiated and undifferentiated spermatogonial stem cells. Biologia Futura. 2022;73(4):503–12.
Amirian M, et al. VASA protein and gene expression analysis of human non-obstructive azoospermia and normal by immunohistochemistry, immunocytochemistry, and bioinformatics analysis. Scientific Rep. 2022;12(1):17259.
Hashemi Karoii D, Azizi H. A review of protein-protein interaction and signaling pathway of Vimentin in cell regulation, morphology and cell differentiation in normal cells. J Recept Signal Transduct. 2022;42(5):512–20.
Reza E, Azizi H. Comparing the expression levels of alkaline phosphatase, Gfra1, Lin28, and Sall4 Genes in embryonic stem cells, spermatogonial stem cells, and embryonic stem-like cells in mice. J Maz Univ Med Sci. 2022;32(210):13–25.
Reza E, Azizi H, Ahmadi AA. Evaluation and comparison of the expression levels of the ZBTB16 (Plzf) and ZFP genes and alkaline phosphatase in three cell populations: mouse spermatogonial stem cells, embryonic stem-like cells (Es-like), and embryonic stem cells. J Ilam Univ Med Sci. 2023;31(1):1.
Tesar PJ, et al. New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nat. 2007;448(7150):196–9.
Narisawa S, et al. Accelerated fat absorption in intestinal alkaline phosphatase knockout mice. Mol Cell Biol. 2003;23(21):7525–30.
Singh U, et al. Novel live alkaline phosphatase substrate for identification of pluripotent stem cells. Stem Cell Rev Rep. 2012;8(3):1021–9.
Tan H, Tee WW. Committing the primordial germ cell: an updated molecular perspective. Wiley Interdiscip Rev: Syst Biol Med. 2019;11(1):e1436.
González F, Boué S, Belmonte JCI. Methods for making induced pluripotent stem cells: reprogramming a la carte. Nat Rev Genet. 2011;12(4):231–42.
Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7(1):07002.
He S, et al. Developmental expression of pluripotency determining factors in caprine embryos: novel pattern of NANOG protein localization in the nucleolus. Mol Reprod Dev. 2006;73(12):1512–22.
Carlin R, et al. Expression of early transcription factors Oct-4, Sox-2 and Nanog by porcine umbilical cord (PUC) matrix cells. Reprod Biol Endocrinol. 2006;4(1):1–13.
Goel S, et al. Expression of NANOG, but not POU5F1, points to the stem cell potential of primitive germ cells in neonatal pig testis. Reproduction. 2008;135(6):785.
Zhang L, et al. Successful co-immunoprecipitation of Oct4 and Nanog using cross-linking. Biochem Biophys Res Commun. 2007;361(3):611–4.
Sánchez-Sánchez AV, et al. Nanog regulates primordial germ cell migration through Cxcr4b. Stem Cells. 2010;28(9):1457–64.
Yu M, et al. Maternal inheritance of Nanog ortholog in blunt-snout bream. J Exp Zool B Mol Dev Evol. 2017;328(8):749–59.
Liu M, de Mitcheson YS. Gonad development during sexual differentiation in hatchery-produced orange-spotted grouper (Epinephelus coioides) and humpback grouper (Cromileptes altivelis)(Pisces: Serranidae, Epinephelinae). Aquaculture. 2009;287(1-2):191–202.
Gao J, et al. Identification and characterization of a Nanog homolog in Japanese flounder (Paralichthys olivaceus). Gene. 2013;531(2):411–21.
Azizi H, et al. Derivation of pluripotent cells from mouse SSCs seems to be age dependent. Stem cells int. 2016;2016:8216312.
Hochedlinger K, et al. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121(3):465–77.
Nowak-Imialek M, et al. Oct4-enhanced green fluorescent protein transgenic pigs: a new large animal model for reprogramming studies. Stem Cells and Dev. 2011;20(9):1563–75.
Campolo F, et al. Essential role of Sox2 for the establishment and maintenance of the germ cell line. Stem cells. 2013;31(7):1408–21.
Conrad S, et al. Differential gene expression profiling of enriched human spermatogonia after short-and long-term culture. BioMed res int. 2014;2014:138350.
Muhr J. Genomic occupancy in various cellular contexts and potential pioneer factor function of SOX2. In: Sox2. Elsevier; 2016. p. 145–59.
Yu Z, et al. Gene expression profiles in different stages of mouse spermatogenic cells during spermatogenesis. Biology of reprod. 2003;69(1):37–47.
Cannarella R, et al. Molecular biology of spermatogenesis: novel targets of apparently idiopathic male infertility. Int J Mol Sci. 2020;21(5):1728.
Acknowledgements
The faculty of biotechnology of Amol University of Special Modern Technologies, Islamic Republic of Iran, and the University of Heidelberg, Institute supported this research for Anatomy and Cell Biology III, Department of Neuroanatomy, Germany.
Funding
This research has been done with the financial support of the Centre for International Scientific Studies and Collaboration (CISSC) of the Ministry of Science, Research and Technology as well as the Iranian National Science Foundation (INSF) under project No.4006239, and a memorandum of understanding (MOU) agreement between the Institute for Anatomy and Cell Biology at the University of Heidelberg and Amol University of Special Modern Technology.
Author information
Authors and Affiliations
Contributions
ER: designed the bioinformatics data, wrote the manuscript, and did the data analysis. HA: accomplished and design the experiment, assembly of data, and data analysis. The authors read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethical Consideration
In the current investigation, animal experiments were approved (Ir.ausmt.rec.1400.29.) by the Ethics Committee of Amol University of Special Modern Technologies.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Description of Future Works
In the future, it is targeted that by knowing the relationship and effects of factors and also by examining the differentiation conditions of spermatogonial stem cells or pluripotent sources such as ES-like cells, mature and fertile sperm cells can be created for the treatment of infertility.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Reza, E., Azizi, H. Correlation Between Alkaline Phosphatase Expression and Sox2, Oct4, and Nanog Genes in Spermatogonial and ES-Like Cells. Regen. Eng. Transl. Med. (2023). https://doi.org/10.1007/s40883-023-00316-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s40883-023-00316-y