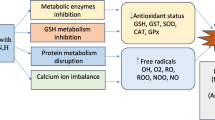
Abstract
Aluminum contamination is a growing environmental and public health concern, and aluminum testicular toxicity has been reported in male rats; however, the underlying mechanisms of this toxicity are unclear. The objective of this study was to investigate the effects of exposure to aluminum chloride (AlCl3) on alterations in the levels of sex hormones (testosterone [T], luteinizing hormone [LH], and follicle-stimulating hormone [FSH]) and testicular damage. Additionally, the mechanisms of toxicity in the testes of AlCl3-exposed rats were analyzed by proteomics. Three different concentrations of AlCl3 were administered to rats. The results demonstrated a decrease in T, LH, and FSH levels with increasing concentrations of AlCl3 exposure. HE staining results revealed that the spermatogenic cells in the AlCl3-exposed rats were widened, disorganized, or absent, with increased severe tissue destruction at higher concentrations of AlCl3 exposure. Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) enrichment analyses revealed that differentially expressed proteins (DEPs) after AlCl3 exposure were primarily associated with various metabolic processes, sperm fibrous sheath, calcium-dependent protein binding, oxidative phosphorylation, and ribosomes. Subsequently, DEPs from each group were subjected to protein-protein interaction (PPI) analysis followed by the screening of interactional key DEPs. Western blot experiments validated the proteomics data, revealing the downregulation of sperm-related DEPs (AKAP4, ODF1, and OAZ3) and upregulation of regulatory ribosome-associated protein (UBA52) and mitochondrial ribosomal protein (MRPL32). These findings provide a basis for studying the mechanism of testicular toxicity due to AlCl3 exposure.










Similar content being viewed by others
Data Availability
All data will be provided upon request.
References
Chen H, Chow CL, Lau D (2022) Deterioration mechanisms and advanced inspection technologies of aluminum windows. Materials 15:354. https://doi.org/10.3390/ma15010354
Chauhan DK, Yadav V, Vaculík M et al (2021) Aluminum toxicity and aluminum stress-induced physiological tolerance responses in higher plants. Crit Rev Biotechnol 41:715–730. https://doi.org/10.1080/07388551.2021.1874282
Liu Q, Zhou L, Liu F et al (2019) Uptake and subcellular distribution of aluminum in a marine diatom. Ecotoxicol Environ Saf 169:85–92. https://doi.org/10.1016/j.ecoenv.2018.10.095
Shetty R, Vidya CS-N, Prakash NB et al (2021) Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: a review. Sci Total Environ 765:142744. https://doi.org/10.1016/j.scitotenv.2020.142744
Wang D, He Y, Liang J et al (2013) Distribution and source analysis of aluminum in rivers near Xi’an City, China. Environ Monit Assess 185:1041–1053. https://doi.org/10.1007/s10661-012-2612-2
Makhdoomi S, Ariafar S, Mirzaei F, Mohammadi M (2023) Aluminum neurotoxicity and autophagy: a mechanistic view. Neurol Res 45:216–225. https://doi.org/10.1080/01616412.2022.2132727
Ahmed WMS, Ibrahim MA, Helmy NA et al (2022) Amelioration of aluminum-induced hepatic and nephrotoxicity by Premna odorata extract is mediated by lowering MMP9 and TGF-β gene alterations in Wistar rat. Environ Sci Pollut Res 29:72827–72838. https://doi.org/10.1007/s11356-022-20735-8
Zhou L, He M, Li X et al (2022) Molecular mechanism of aluminum-induced oxidative damage and apoptosis in rat cardiomyocytes. Biol Trace Elem Res 200:308–317. https://doi.org/10.1007/s12011-021-02646-w
Yuan H-X, Pang Y-F, Wang J-L, Chen W-C (2019) Impacts of aluminum on sperm quality and sperm mitochondria in male rats. Zhonghua Nan Ke Xue 25:579–585
Miska-Schramm A, Kapusta J, Kruczek M (2017) The effect of aluminum exposure on reproductive ability in the bank vole (Myodes glareolus). Biol Trace Elem Res 177:97–106. https://doi.org/10.1007/s12011-016-0848-3
Cheraghi E, Golkar A, Roshanaei K, Alani B (2017) Aluminium-induced oxidative stress, apoptosis and alterations in testicular tissue and sperm quality in Wistar rats: ameliorative effects of curcumin. Int J fertil Steril 11. https://doi.org/10.22074/ijfs.2017.4859
da Silva LD, da Silva GL, de Sousa FE et al (2020) Aluminum exposure promotes histopathological and pro-oxidant damage to the prostate and gonads of male and female adult gerbils. Exp Mol Pathol 116:104486. https://doi.org/10.1016/j.yexmp.2020.104486
Rozanova S, Barkovits K, Nikolov M et al (2021) Quantitative mass spectrometry-based proteomics: an overview. Methods Mol Biol 2228:85–116. https://doi.org/10.1007/978-1-0716-1024-4_8
McArdle AJ, Menikou S (2021) What is proteomics? Arch Dis Child Educ Pract Ed 106:178–181. https://doi.org/10.1136/archdischild-2019-317434
Liu Z, Li Y, Sepúlveda MS et al (2021) Development of an adverse outcome pathway for nanoplastic toxicity in Daphnia pulex using proteomics. Sci Total Environ 766:144249. https://doi.org/10.1016/j.scitotenv.2020.144249
Sun X, Wang Y, Jiang T et al (2021) Nephrotoxicity profile of cadmium revealed by proteomics in mouse kidney. Biol Trace Elem Res 199:1929–1940. https://doi.org/10.1007/s12011-020-02312-7
Yurchenko VV, Morozov AA, Kiriukhin BA (2022) Proteomics analysis in Japanese Medaka Oryzias latipes exposed to humic acid revealed suppression of innate immunity and coagulation proteins. Biology (Basel) 11:683. https://doi.org/10.3390/biology11050683
Khan ZN, Sabino IT, de Souza Melo CG et al (2019) Liver proteome of mice with distinct genetic susceptibilities to fluorosis treated with different concentrations of F in the drinking water. Biol Trace Elem Res 187:107–119. https://doi.org/10.1007/s12011-018-1344-8
Xu F, Liu Y, Zhao H et al (2017) Aluminum chloride caused liver dysfunction and mitochondrial energy metabolism disorder in rat. J Inorg Biochem 174:55–62. https://doi.org/10.1016/j.jinorgbio.2017.04.016
Doyle TJ, Oudes AJ, Kim KH (2009) Temporal profiling of rat transcriptomes in retinol-replenished vitamin A-deficient testis. Syst Biol Reprod Med 55:145–163. https://doi.org/10.3109/19396360902896844
Yang Y, Luo J, Yu D et al (2018) Vitamin A promotes Leydig cell differentiation via alcohol dehydrogenase 1. Front Endocrinol 9:644. https://doi.org/10.3389/fendo.2018.00644
Béziers P, Ducrest A-L, Simon C, Roulin A (2017) Circulating testosterone and feather-gene expression of receptors and metabolic enzymes in relation to melanin-based colouration in the barn owl. Gen Comp Endocrinol 250:36–45. https://doi.org/10.1016/j.ygcen.2017.04.015
Topo E, Soricelli A, D’Aniello A et al (2009) The role and molecular mechanism of D-aspartic acid in the release and synthesis of LH and testosterone in humans and rats. Reprod Biol Endocrinol 7:120. https://doi.org/10.1186/1477-7827-7-120
Santillo A, Falvo S, Chieffi P et al (2016) D-aspartate induces proliferative pathways in spermatogonial GC-1 cells: GC-1 CELL PROLIFERATION INDUCED BY D-Asp. J Cell Physiol 231:490–495. https://doi.org/10.1002/jcp.25095
Morris MB, Ozsoy S, Zada M et al (2020) Selected amino acids promote mouse pre-implantation embryo development in a growth factor-like manner. Front Physiol 11:140. https://doi.org/10.3389/fphys.2020.00140
Ma C, Mirth CK, Hall MD, Piper MDW (2022) Amino acid quality modifies the quantitative availability of protein for reproduction in Drosophila melanogaster. J Insect Physiol 139:104050. https://doi.org/10.1016/j.jinsphys.2020.104050
Ommati MM, Heidari R, Zamiri MJ et al (2020) The footprints of oxidative stress and mitochondrial impairment in arsenic trioxide-induced testosterone release suppression in pubertal and mature F1-male Balb/c mice via the downregulation of 3β-HSD, 17β-HSD, and CYP11a expression. Biol Trace Elem Res 195:125–134. https://doi.org/10.1007/s12011-019-01815-2
Oduwole OO, Peltoketo H, Huhtaniemi IT (2018) Role of follicle-stimulating hormone in spermatogenesis. Front Endocrinol (Lausanne) 9:763. https://doi.org/10.3389/fendo.2018.00763
Ozcan Yildirim S, Colakoglu N, Ozer Kaya S (2022) Protective effects of L -arginine against aluminium chloride-induced testicular damage in rats. Andrologia 54. https://doi.org/10.1111/and.14569
Gao D-D, Lan C-F, Cao X-N et al (2022) G protein-coupled estrogen receptor promotes acrosome reaction via regulation of Ca2+ signaling in mouse sperm†. Biol Reprod 107:1026–1034. https://doi.org/10.1093/biolre/ioac136
Sato T, Arimura T, Murata K et al (2021) Differences of extracellular cues and Ca2+ permeable channels in the signaling path differences ways for inducing amphibian sperm motility. Zoolog Sci 38:343–351. https://doi.org/10.2108/zs200159
Zhou F, Du G, Xie J et al (2020) RyRs mediate lead-induced neurodegenerative disorders through calcium signaling pathways. Sci Total Environ 701:134901. https://doi.org/10.1016/j.scitotenv.2019.134901
Ren T, Tang Y, Wang M et al (2020) Triptolide induces apoptosis through the calcium/calmodulin-dependent protein kinase kinaseβ/AMP-activated protein kinase signaling pathway in non-small cell lung cancer cells. Oncol Rep. https://doi.org/10.3892/or.2020.7763
Ham J, Lim W, You S, Song G (2020) Butylated hydroxyanisole induces testicular dysfunction in mouse testis cells by dysregulating calcium homeostasis and stimulating endoplasmic reticulum stress. Sci Total Environ 702:134775. https://doi.org/10.1016/j.scitotenv.2019.134775
Li Y, Jin L, Li Y et al (2022) Lysophosphatidic acid improves human sperm motility by enhancing glycolysis and activating L-type calcium channels. Front Endocrinol (Lausanne) 13:896558. https://doi.org/10.3389/fendo.2022.896558
Liu X, Teng Z, Wang Z et al (2022) Expressions of HSPA1L and HSPA9 are associated with poor sperm quality of low-motility spermatozoa in fertile men. Andrologia 54:e14321. https://doi.org/10.1111/and.14321
Park Y-J, Pang M-G (2021) Mitochondrial functionality in male fertility: from spermatogenesis to fertilization. Antioxidants (Basel) 10:98. https://doi.org/10.3390/antiox10010098
Tang W, Xiao Y, Long Y et al (2021) Sodium fluoride causes oxidative damage to silkworm (Bombyx mori) testis by affecting the oxidative phosphorylation pathway. Ecotoxicol Environ Saf 218:112229. https://doi.org/10.1016/j.ecoenv.2021.112229
da Silva J, Gonçalves RV, de Melo FCSA et al (2021) Cadmium Exposure and testis susceptibility: a systematic review in murine models. Biol Trace Elem Res 199:2663–2676. https://doi.org/10.1007/s12011-020-02389-0
Shih H-J, Chang C-Y, Huang I-T et al (2021) Testicular torsion-detorsion causes dysfunction of mitochondrial oxidative phosphorylation. Andrology 9:1902–1910. https://doi.org/10.1111/andr.13068
Dibley MG, Formosa LE, Lyu B et al (2020) The mitochondrial acyl-carrier protein interaction network highlights important roles for LYRM family members in complex I and mitoribosome assembly. Mol Cell Proteomics 19:65–77. https://doi.org/10.1074/mcp.RA119.001784
Zhang R, Hou T, Cheng H, Wang X (2019) NDUFAB1 protects against obesity and insulin resistance by enhancing mitochondrial metabolism. FASEB J 33:13310–13322. https://doi.org/10.1096/fj.201901117RR
Hou T, Zhang R, Jian C et al (2019) NDUFAB1 confers cardio-protection by enhancing mitochondrial bioenergetics through coordination of respiratory complex and supercomplex assembly. Cell Res 29:754–766. https://doi.org/10.1038/s41422-019-0208-x
Chakraborty B, Bhakta S, Sengupta J (2016) Mechanistic insight into the reactivation of BCAII enzyme from denatured and molten globule states by eukaryotic ribosomes and domain V rRNAs. PLoS One 11:e0153928. https://doi.org/10.1371/journal.pone.0153928
Branco AT, Lemos B (2014) High intake of dietary sugar enhances bisphenol A (BPA) disruption and reveals ribosome-mediated pathways of toxicity. Genetics 197:147–157. https://doi.org/10.1534/genetics.114.163170
Shen X, Yin L, Pan X et al (2020) Porcine epidemic diarrhea virus infection blocks cell cycle and induces apoptosis in pig intestinal epithelial cells. Microb Pathog 147:104378. https://doi.org/10.1016/j.micpath.2020.104378
Huang G, Li H, Zhang H (2020) Abnormal expression of mitochondrial ribosomal proteins and their encoding genes with cell apoptosis and diseases. IJMS 21:8879. https://doi.org/10.3390/ijms21228879
Guan X, Zhang H, Qin H et al (2020) CRISPR/Cas9-mediated whole genomic wide knockout screening identifies mitochondrial ribosomal proteins involving in oxygen-glucose deprivation/reperfusion resistance. J Cell Mol Med 24:9313–9322. https://doi.org/10.1111/jcmm.15580
Zhou Q, Hou Z, Zuo S et al (2019) LUCAT1 promotes colorectal cancer tumorigenesis by targeting the ribosomal protein L40- MDM 2-p53 pathway through binding with UBA 52. Cancer Sci 110:1194–1207. https://doi.org/10.1111/cas.13951
Carracedo S, Briand-Amirat L, Dordas-Perpinyà M et al (2022) ProAKAP4 protein marker: towards a functional approach to male fertility. Anim Reprod Sci 247:107074. https://doi.org/10.1016/j.anireprosci.2022.107074
Fang X, Huang L-L, Xu J et al (2019) Proteomics and single-cell RNA analysis of Akap4-knockout mice model confirm indispensable role of Akap4 in spermatogenesis. Dev Biol 454:118–127. https://doi.org/10.1016/j.ydbio.2019.06.017
Zhao W, Li Z, Ping P et al (2018) Outer dense fibers stabilize the axoneme to maintain sperm motility. J Cell Mol Med 22:1755–1768. https://doi.org/10.1111/jcmm.13457
Hoyer-Fender S (2022) Development of the connecting piece in ODF1-deficient mouse spermatids. IJMS 23:10280. https://doi.org/10.3390/ijms231810280
Sarkar S, Yadav S, Mehta P et al (2022) Histone methylation regulates gene expression in the round spermatids to set the RNA payloads of sperm. Reprod Sci 29:857–882. https://doi.org/10.1007/s43032-021-00837-3
Paclik D, Danese S, Berndt U et al (2008) Galectin-4 controls intestinal inflammation by selective regulation of peripheral and mucosal T cell apoptosis and cell cycle. PLoS One 3:e2629. https://doi.org/10.1371/journal.pone.0002629
Cao Z-Q, Guo X-L (2016) The role of galectin-4 in physiology and diseases. Protein Cell 7:314–324. https://doi.org/10.1007/s13238-016-0262-9
Funding
We thank the Guangxi Natural Science Foundation Project (2020GXNSFAA297257), Guangxi Science and Technology Program Project (21-220-22), Guangxi University Young and Middle-aged Teachers’ Basic Ability Improvement Project (2020KY13017), Guangxi Zhuang Autonomous Region Administration of Traditional Chinese Medicine Self-financing Scientific Research Project (GZZC2020248), and Guangxi Zhuang Autonomous Region Health and Health Commission Self-financing Scientific Research Course (Z20201416).
Author information
Authors and Affiliations
Contributions
Huixin Peng, Yanxin Huang, and Guangji Wei were responsible for experiment operation and paper writing; Yanfang Pang and Huixiong Yuan were responsible for animal feeding and modeling; Xiong Zou was responsible for partial data analysis; Wencheng Chen and Yu’an Xie were responsible for experiment design, paper writing guidance, overall framework construction, and project fund preparation.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, H., Huang, Y., Wei, G. et al. Testicular Toxicity in Rats Exposed to AlCl3: a Proteomics Study. Biol Trace Elem Res 202, 1084–1102 (2024). https://doi.org/10.1007/s12011-023-03745-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-023-03745-6