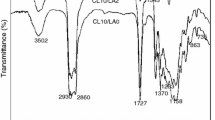
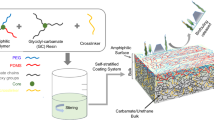
Abstract
Existing antifouling coatings for marine environments rely on the toxicity of copper and small-molecule additives to prevent organism growth. These come with a myriad of environmental and health-related risks, as these additives inevitably leach into seawater. Enzyme-based antifouling polymers have tremendous potential as part of an environmentally innocuous antifouling strategy, but have not yet been commercially realized. Biofouling begins with small molecules and polymers that initiate settlement for larger organisms. Using an enzymatic coating to rapidly hydrolyze these compounds could reduce the surface concentration of these attractive polymers and prevent organism growth. Here, antifouling xylanase and lysing complex enzymes were covalently tethered to surfaces using isoindolinone groups and click chemistry. We found that xylanase and lysing complex continue to hydrolyze xylosidic bonds in hemicellulose polysaccharides after covalent tethering, and the coating maintained activities of > 80% and > 50%, respectively, after 2 months while submerged in artificial seawater, demonstrating this material’s potential as an eco-friendly antifouling coating.
Graphical abstract





Similar content being viewed by others
References
Pasmore, M, Costerton, JW, “Biofilms, Bacterial Signaling, and Their Ties to Marine Biology.” J. Ind. Microbiol. Biotechnol., 30 (7) 407–413. https://doi.org/10.1007/s10295-003-0069-6 (2003)
Molino, PJ, Wetherbee, R, “The Biology of Biofouling Diatoms and Their Role in the Development of Microbial Slimes.” Biofouling, 24 (5) 365–379. https://doi.org/10.1080/08927010802254583 (2008)
Buskens, P, Wouters, M, Rentrop, C, Vroon, Z, “A Brief Review of Environmentally Benign Antifouling and Foul-Release Coatings for Marine Applications.” J. Coat. Technol. Res., 10 (1) 29–36. https://doi.org/10.1007/s11998-012-9456-0 (2013)
Briand, J-F, “Marine Antifouling Laboratory Bioassays: An Overview of Their Diversity.” Biofouling, 25 (4) 297–311. https://doi.org/10.1080/08927010902745316 (2009)
Royer, S-J, Wiggin, K, Kogler, M, Deheyn, DD, “Degradation of Synthetic and Wood-Based Cellulose Fabrics in the Marine Environment: Comparative Assessment of Field, Aquarium, and Bioreactor Experiments.” Sci. Total Environ., 791 148060. https://doi.org/10.1016/j.scitotenv.2021.148060 (2021)
Kyei, SK, Darko, G, Akaranta, O, “Chemistry and Application of Emerging Ecofriendly Antifouling Paints: A Review.” J. Coat. Technol. Res., 17 (2) 315–332. https://doi.org/10.1007/s11998-019-00294-3 (2020)
Yebra, DM, Kiil, S, Dam-Johansen, K, “Antifouling Technology—Past, Present and Future Steps Towards Efficient and Environmentally Friendly Antifouling Coatings.” Prog. Org. Coat., 50 (2) 75–104. https://doi.org/10.1016/j.porgcoat.2003.06.001 (2004)
Aykin, E, Omuzbuken, B, Kacar, A, “Microfouling Bacteria and the Use of Enzymes in Eco-Friendly Antifouling Technology.” J. Coat. Technol. Res., 16 (3) 847–856. https://doi.org/10.1007/s11998-018-00161-7 (2019)
Cordeiro, AL, Werner, C, “Enzymes for Antifouling Strategies.” J. Adhes. Sci. Technol., 25 (17) 2317–2344. https://doi.org/10.1163/016942411X574961 (2011)
Lee, J, Lee, I, Nam, J, Hwang, DS, Yeon, K-M, Kim, J, “Immobilization and Stabilization of Acylase on Carboxylated Polyaniline Nanofibers for Highly Effective Antifouling Application via Quorum Quenching.” ACS Appl. Mater. Interfaces, 9 (18) 15424–15432. https://doi.org/10.1021/acsami.7b01528 (2017)
Olsen, SM, Pedersen, LT, Laursen, MH, Kiil, S, Dam-Johansen, K, “Enzyme-Based Antifouling Coatings: A Review.” Biofouling, 23 (5) 369–383. https://doi.org/10.1080/08927010701566384 (2007)
Zhang, Y, Liang, Y, Huang, F, Zhang, Y, Li, X, Xia, J, “Site-Selective Lysine Reactions Guided by Protein-Peptide Interaction.” Biochemistry, 58 (7) 1010–1018. https://doi.org/10.1021/acs.biochem.8b01223 (2019)
Tung, CL, Wong, CTT, Fung, EYM, Li, X, “Traceless and Chemoselective Amine Bioconjugation via Phthalimidine Formation in Native Protein Modification.” Org. Lett., 18 (11) 2600–2603. https://doi.org/10.1021/acs.orglett.6b00983 (2016)
Zhang, Q, Zhang, Y, Liu, H, Chow, HY, Tian, R, Eva Fung, YM, Li, X, “OPA-Based Bifunctional Linker for Protein Labeling and Profiling.” Biochemistry, 59 (2) 175–178. https://doi.org/10.1021/acs.biochem.9b00787 (2020)
Pickens, CJ, Johnson, SN, Pressnall, MM, Leon, MA, Berkland, CJ, “Practical Considerations, Challenges, and Limitations of Bioconjugation via Azide-Alkyne Cycloaddition.” Bioconjug. Chem., 29 (3) 686–701. https://doi.org/10.1021/acs.bioconjchem.7b00633 (2018)
Bartlett, ME, Shuler, SA, Rose, DJ, Gilbert, LM, Hegab, RA, Lawton, TJ, Messersmith, RE, “Paintable Proteins: Biofunctional Coatings via Covalent Incorporation of Proteins into a Polymer Network.” New J. Chem., 45 (47) 22084–22092. https://doi.org/10.1039/D1NJ04687J (2021)
Wine, Y, Cohen-Hadar, N, Freeman, A, Frolow, F, “Elucidation of the Mechanism and End Products of Glutaraldehyde Crosslinking Reaction by X-Ray Structure Analysis.” Biotechnol. Bioeng., 98 (3) 711–718. https://doi.org/10.1002/bit.21459 (2007)
Hu, J, Davies, J, Mok, YK, Arato, C, Saddler, JN, “The Potential of Using Immobilized Xylanases to Enhance the Hydrolysis of Soluble, Biomass Derived Xylooligomers.” Materials, 11 (10) 2005 (2018)
Kapoor, M, Kuhad, RC, “Immobilization of Xylanase from Bacillus pumilus Strain MK001 and its Application in Production of Xylo-oligosaccharides.” Appl. Biochem. Biotechnol., 142 (2) 125–138. https://doi.org/10.1007/s12010-007-0013-8 (2007)
Pal, A, Khanum, F, “Covalent Immobilization of Xylanase on Glutaraldehyde Activated Alginate Beads Using Response Surface Methodology: Characterization of Immobilized Enzyme.” Process Biochem., 46 (6) 1315–1322. https://doi.org/10.1016/j.procbio.2011.02.024 (2011)
Hu, J, Davies, J, Mok, YK, Arato, C, Saddler, JN, “The Potential of Using Immobilized Xylanases to Enhance the Hydrolysis of Soluble, Biomass Derived Xylooligomers.” Materials, 11 (10) 2005. https://doi.org/10.3390/ma11102005 (2018)
Kumar, L, Nagar, S, Mittal, A, Garg, N, Gupta, VK, “Immobilization of Xylanase Purified from Bacillus pumilus VLK-1 and its Application in Enrichment of Orange and Grape Juices.” J. Food Sci. Technol., 51 (9) 1737–1749. https://doi.org/10.1007/s13197-014-1268-z (2014)
Hlady, V, Buijs, J, “Protein Adsorption on Solid Surfaces.” Curr. Opin. Biotechnol., 7 (1) 72–77. https://doi.org/10.1016/S0958-1669(96)80098-X (1996)
Rabe, M, Verdes, D, Seeger, S, “Understanding Protein Adsorption Phenomena at Solid Surfaces.” Adv. Colloid Interface Sci., 162 (1) 87–106. https://doi.org/10.1016/j.cis.2010.12.007 (2011)
Le Droumaguet, B, Velonia, K, “Click Chemistry: A Powerful Tool to Create Polymer-Based Macromolecular Chimeras.” Macromol. Rapid Commun., 29 (12–13) 1073–1089. https://doi.org/10.1002/marc.200800155 (2008)
Thordarson, P, Le Droumaguet, B, Velonia, K, “Well-Defined Protein–Polymer Conjugates—Synthesis and Potential Applications.” Appl. Microbiol. Biotechnol., 73 (2) 243–254. https://doi.org/10.1007/s00253-006-0574-4 (2006)
Fontaine, SD, Reid, R, Robinson, L, Ashley, GW, Santi, DV, “Long-Term Stabilization of Maleimide-Thiol Conjugates.” Bioconjugate Chem., 26 (1) 145–152. https://doi.org/10.1021/bc5005262 (2015)
Weltz, JS, Kienle, DF, Schwartz, DK, Kaar, JL, “Reduced Enzyme Dynamics Upon Multipoint Covalent Immobilization Leads to Stability-Activity Trade-off.” J. Am. Chem. Soc., 142 (7) 3463–3471. https://doi.org/10.1021/jacs.9b11707 (2020)
Bey, H, Gtari, W, Aschi, A, Othman, T, “Structure and Properties of Native and Unfolded Lysing Enzyme from T. Harzianum: Chemical and pH Denaturation.” Int. J. Biol. Macromol., 92 860–866. https://doi.org/10.1016/j.ijbiomac.2016.08.001 (2016)
Horta, MAC, Filho, JAF, Murad, NF, de Oliveira Santos, E, dos Santos, CA, Mendes, JS, Brandão, MM, Azzoni, SF, de Souza, AP, “Network of Proteins, Enzymes and Genes Linked to Biomass Degradation Shared by Trichoderma Species.” Sci. Rep., 8 (1) 1341. https://doi.org/10.1038/s41598-018-19671-w (2018)
Kristensen, JB, Meyer, RL, Laursen, BS, Shipovskov, S, Besenbacher, F, Poulsen, CH, “Antifouling Enzymes and the Biochemistry of Marine Settlement.” Biotechnol. Adv., 26 (5) 471–481. https://doi.org/10.1016/j.biotechadv.2008.05.005 (2008)
Acknowledgments
Johns Hopkins University Applied Physics Laboratory Research and Exploratory Development Department provided internal research funding for this work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baker-Branstetter, R.W., Bartlett, M.E., Shuler, S.A. et al. Covalent immobilization of xylanase and lysing complex into polymer scaffolds with long-term activity retention. J Coat Technol Res 20, 973–978 (2023). https://doi.org/10.1007/s11998-022-00717-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11998-022-00717-8