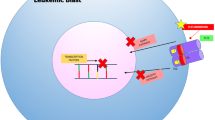
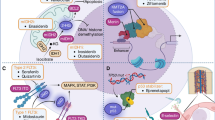
Abstract
Purpose of Review
Acute myeloid leukemia (AML) is an aggressive malignancy of the bone marrow that has a poor prognosis with traditional cytotoxic chemotherapy, especially in elderly patients. In recent years, small molecule inhibitors targeting AML-associated IDH1, IDH2, and FLT3 mutations have been FDA approved. However, the majority of AML cases do not have a targetable mutation. A variety of novel agents targeting both previously untargetable mutations and general pathways in AML are currently being investigated. Herein, we review selected new targeted therapies currently in early-phase clinical investigation in AML.
Recent Findings
The DOT1L inhibitor pinometostat in KMT2A-rearranged AML, the menin inhibitors KO-539 and SYNDX-5613 in KMT2Ar and NPM1-mutated AML, and the mutant TP53 inhibitor APR-246 are examples of novel agents targeting specific mutations in AML. In addition, BET inhibitors, polo-like kinase inhibitors, and MDM2 inhibitors are promising new drug classes for AML which do not depend on the presence of a particular mutation.
Summary
AML remains in incurable disease for many patients but advances in genomics, epigenetics, and drug discovery have led to the development of many potential novel therapeutic agents, many of which are being investigated in ongoing clinical trials. Additional studies will be necessary to determine how best to incorporate these novel agents into routine clinical treatment of AML.
Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Döhner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–52. https://doi.org/10.1056/NEJMra1406148.
Ley TJ, Miller C, Ding L, Raphael BJ, Mungall AJ, Robertson AG, et al. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med. 2013;368:2059–74. https://doi.org/10.1056/NEJMoa1301689.
Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016;374:2209–21. https://doi.org/10.1056/NEJMoa1516192.
Eisfeld AK, Mrόzek K, Kohlschmidt J, Nicolet D, Orwick S, Walker CJ, et al. The mutational oncoprint of recurrent cytogenetic abnormalities in adult patients with de novo acute myeloid leukemia. Leukemia. 2017;31:2211–8. https://doi.org/10.1038/leu.2017.86.
Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood. 2017;130:722–31. https://doi.org/10.1182/blood-2017-03-779405.
Pollyea DA, Tallman MS, de Botton S, Kantarjian HM, Collins R, Stein AS, et al. Enasidenib, an inhibitor of mutant IDH2 proteins, induces durable remissions in older patients with newly diagnosed acute myeloid leukemia. Leukemia. 2019;33:2575–84. https://doi.org/10.1038/s41275-019-0472-2.
DiNardo CD, Stein EM, do Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable remissions with ivosidenib in IDH1-mutated relapsed or refractory AML. N Engl J Med. 2018;378:2386–98. https://doi.org/10.1056/NEJMoa1716984.
Roboz GJ, DiNardo CD, Stein EM, de Botton S, Mims AS, Prince GT, et al. Ivosidenib induces deep durable remissions in patients with newly diagnosed IDH1-mutant acute myeloid leukemia. Blood. 2020;135:463–71. https://doi.org/10.1182/blood.2019002140.
Stone RM, Mandrekar SJ, Sanford BL, Laumann K, Geyer S, Bloomfield CD, et al. Midostaurin plus chemotherapy for acute myeloid leukemia with a FLT3 mutation. N Engl J Med. 2017;377:454–64. https://doi.org/10.1056/NEJMoa1614359.
Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or chemotherapy for relapsed or refractory FLT3-mutated AML. N Engl J Med. 2019;381:1728–40. https://doi.org/10.1056/NEJMoa1902688.
Wei AH, Montensinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135:2137–45. https://doi.org/10.1182/blood.2020004856.
DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020;383:617–29. https://doi.org/10.1056/NEJMoa2012971.
Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia. 2019;33:379–89. https://doi.org/10.1038/s41375-018-0312-9.
Zuber J, Shi J, Wang E, Rappaport AR, Herrmann H, Sison EA, et al. RNAi screen identifies Brd4 as a therapeutic target in acute myeloid leukemia. Nature. 2011;478:524–8. https://doi.org/10.1038/nature10334.
Herrmann H, Blatt K, Shi J, Gleixner KV, Cerny-Reiterer S, Mȕllauer L, et al. Small-molecule inhibition of BRD4 as a new potent approach to eliminate leukemic stem- and progenitor cells in acute myeloid leukemia AML. Oncotarget. 2012;3(12):1588–99. https://doi.org/10.18632/oncotarget.733.
Pericole FV, Lazarini M, de Paiva LB, Duarte ADSS, Ferro KPV, Niemann FS, et al. BRD4 inhibition enhances azacitidine efficacy in acute myeloid leukemia and myelodysplastic syndromes. Front Oncol. 2019;9:16. https://doi.org/10.3389/fonc.2019.00016.
Tzelepis K, De Braekeleer E, Aspris D, Barbieri I, Vijayabaskar MS, Liu WH, et al. SRPK1 maintains acute myeloid leukemia through effects on isoform usage of epigenetic regulators including BRD4. Nat Commun. 2018;9:5378. https://doi.org/10.1038/s41467-018-07620-0.
• Roe JS, Vakoc CR. The essential transcriptional function of BRD4 in acute myeloid leukemia. Cold Spring Harb Symp Quant Biol. 2016;81:61–6. https://doi.org/10.1101/sqb.2016.81.031039. An excellent review of the function of BRD4 in AML.
Dawson MA, Prinjha RK, Dittmann A, Giotopoulos G, Bantscheff M, Chan WI, et al. Inhibition of BET recruitment to chromatin as an effective treatment for MLL-fusion leukaemia. Nature. 2011;478:529–33. https://doi.org/10.1038/nature10509.
Delmore JE, Issa GC, Lemieux ME, Rahl PB, Shi J, Jacob HM, et al. BET bromodomain inhibition as a therapeutic strategy to target c-Myc. Cell. 2011;146:904–17. https://doi.org/10.1016/j.cell.2011.08.017.
Dawson MA, Gudgin EJ, Horton SJ, Giotopoulos G, Meduri E, Robson S, et al. Recurrent mutations, including NPM1c, activate a BRD4-dependent core transcriptional program in acute myeloid leukemia. Leukemia. 2014;28:311–20. https://doi.org/10.1038/leu.2013.338.
Chen C, Liu Y, Lu C, Cross JR, Morris JP 4th, Shroff AS, et al. Cancer-associated IDH2 mutants drive an acute myeloid leukemia that is susceptible to Brd4 inhibition. Genes Dev. 2013;27:2974–85. https://doi.org/10.1101/gad.226613.113.
Zhang P, He F, Bai J, Yamamoto S, Chen S, Zhang L, et al. Chromatin regulator Asxl1 loss and Nf1 haploinsufficiency cooperate to accelerate myeloid malignancy. J Clin Invest. 2018;128:5383–98. https://doi.org/10.1172/JCI121366.
Zhao Y, Liu Q, Acharya P, Stengel KR, Sheng Q, Zhou X, et al. High-resolution mapping of RNA polymerases identifies mechanisms of sensitivity and resistance to BET inhibitors in t(8;21) AML. Cell Rep. 2016;16:2003–16. https://doi.org/10.1016/j.celrep.2016.07.032.
Saenz DT, Fiskus W, Manshouri T, Rajapakshe K, Krieger S, Sun B, et al. BET protein bromodomain inhibitor-based combinations are highly active against post-myeloproliferative neoplasm secondary AML cells. Leukemia. 2017;31:678–87. https://doi.org/10.1038/leu.2016.260.
Petretich M, Demont E, Grandi P, et al. Domain-selective targeting of BET proteins in cancer and immunological diseases. Curr Opin Chem Biol. 2020;S1367-5931:30019-3. https://doi.org/10.1016/j.cbpa.2020.02.003.
Tyler DS, Vappiani J, Cañeque T, Lam EYN, Ward A, Gilan O, et al. Click chemistry enables preclinical evaluation of targeted epigenetic therapies. Science. 2017;356:1397–401. https://doi.org/10.1126/science.aal2066.
Gilan O, Rioja I, Knezevic K, Bell MJ, Yeung MM, Harker NR, et al. Selective targeting of BD1 and BD2 of the BET proteins in cancer and immunoinflammation. Science. 2020;368:387–94. https://doi.org/10.1126/science.aaz8455.
•• Berthon C, Raffoux E, Thomas X, Vey N, Gomez-Roca C, Yee K, et al. Bromodomain inhibitor OTX015 in patients with acute leukaemia: a dose-escalation, phase 1 study. Lancet Haematol. 2016;3:e186–95. https://doi.org/10.1016/S2352-3026(15)00247-1First clinical trial of a BET inhibitor in acute leukemia.
• Amorim S, Stathis A, Gleeson M, Iyengar S, Magarotto V, Leleu X, et al. Bromodomain inhibitor OTX015 in patients with lymphoma or multiple myeloma: a dose-escalation, open-label, pharmacokinetic, phase 1 study. Lancet Haematol. 2016;3:E196–204. https://doi.org/10.1016/S2452-3026(16)00021-1. The phase 1 study of the BET inhibitor OTX015 in lymphoid malignancies, a companion study to reference 29 above.
Borthakur GM, Odenike O, Aldoss I, Rizzieri DA, Prebet T, Modi DA, et al. Biomarker modulation by mivebresib (ABBV-075) +/- venetoclax in relapsed/refractory acute myeloid leukemia. Blood. 2019;134(Supplement_1):539. https://doi.org/10.1182/blood-2019-126854.
Mascarenhas J, Saab R, Brackman D, Modi DA, Abraham L, Ward JE, et al. Two phase 1b studies evaluating the safety and tolerability of BET inhibitors, ABBV-744 and mivebresib, as monotherapies and in combination with ruxolitinib or navitoclax in patients with myelofibrosis. Blood. 2020;136(Supplement 1):18–9. https://doi.org/10.1182/blood-2020-137686.
Talpaz M, Rampal R, Verstovsek S, Harrison C, Drummond M, Kiladjian JJ, et al. CPI-0610, a bromodomain and extraterminal domain protein (BET) inhibitor, as monotherapy in advanced myelofibrosis patients refractory/intolerant to JAK inhibitor: update from phase 2 Manifest study. European Hematology Association 2020 Annual Meeting. Abstract EP1091.
Mascarenhas J, Harrison C, Ptriarca A, Devos T, Palandri F, Rampal R, et al. CPI-0610, a bromodomain and extraterminal domain protein (BET) inhibitor, in combination with ruxolitinib, in JAK inhibitor treatment naïve myelofibrosis patients: update from the Manifest phase 2 study. European Hematology Association 2020 Annual Meeting. Abstract EP1084.
Verstovsek S, Mascarenhas J, Kremyanskaya M, Hoffman R, Rampal R, Gupta V, et al. CPI-0610, bromodomain and extraterminal domain protein (BET) inhibitor, as ‘add-on’ to ruxolitinib (RUX), in advanced myelofibrosis patients with suboptimal response: update of Manifest phase 2 study. European Hematology Association 2020 Annual Meeting. Abstract EP1083.
Mascarenhas J, Harrison C, Luptakova K, Christo J, Wang J, Mertz JA, et al. MANIFEST-2, a global, phase 3, randomized, double-blind, active-control study of CPI-0610 and ruxolitinib vs. placebo and ruxolitinib in JAK-inhibitor-naive myelofibrosis patients. Blood. 2020;136(Supplement 1):43. https://doi.org/10.1182/blood-2020-140901.
Patel MR, Garcia-Manero G, Paquette R, Dinner S, Donnellan WB, Grunwald MR, et al. Phase 1 dose escalation and expansion study to determine safety, tolerability, pharmacokinetics, and pharmacodynamics of the BET inhibitor FT-1101 as a single agent in patients with relapsed or refractory hematologic malignancies. Blood. 2019;134(Supplement_1):390.
Dawson M, Stein EM, Huntly BJP, Karadimitris A, Kamdar M, de Larrea CF, et al. A phase I study of GSK525762, a selective bromodomain (BRD) and extra terminal protein (BET) inhibitor: results from part 1 of phase I/II open label single agent study in patients with acute myeloid leukemia (AML). Blood. 2017;130(Supplement 1):1377. https://doi.org/10.1182/blood.V130.Suppl_1.1377.1377.
Lihou C, Zhou G, Zheng F. A phase 1 study of INCB057643 monotherapy in patients with relapsed or refractory myelofibrosis (INCB 57643-103). Blood. 2020;136(Supplement 1):16–7. https://doi.org/10.1182/blood-2020-134604.
Mims AS, Solh MD, Borate U, Pemmaraju N, Borthakur G, Roboz GJ, et al. Phase 1b study of BET inhibitor PLX2853 in patients with relapsed or refractory acute myeloid leukemia or high risk myelodysplastic syndrome. Blood. 2020;136(Supplement 1):14–5. https://doi.org/10.1182/blood-2020-140138.
Patnaik A, Carvajal RD, Komatsubara KM, Britten CD, Wesolowski R, Michelson G, et al. Phase ib/2a study of PLX51107, a small molecule BET inhibitor, in subjects with advanced hematological malignancies and solid tumors. J Clin Oncol. 2018;36:2550. https://doi.org/10.1200/JCO.2018.36.15_suppl.2550.
Roboz GJ, Desai P, Lee S, Ritchie EK, Winer ES, DeMario M, et al. A dose escalation study of RO6870810/TEN-10 in patients with acute myeloid leukemia and myelodysplastic syndrome. Leuk Lymphoma 2021;1-13. https://doi.org/10.1080/10428194.2021.1881509. Online ahead of print.
Yang L, Ding L, Liang J, Chen J, Tang Y, Xue H, et al. Relatively favorable prognosis for MLL-rearranged childhood acute leukemia with reciprocal translocations. Pediatr Blood Cancer. 2018;65:e27266. https://doi.org/10.1002/pbc.27266.
Super HJ, McCabe NR, Thirman MJ, Larson RA, Le Beau MM, Pedersen-Bjergaard J, et al. Rearrangements of the MLL gene in therapy-related acute myeloid leukemia in patients previously treated with agents targeting DNA-topoisomerase II. Blood. 1993;83:3705–11.
Chen Y, Kantarjian H, Pierce S, Faderl S, O’Brien S, Qiao W, et al. Prognostic significance of 11q23 aberrations in adult acute myeloid leukemia and the role of allogeneic stem cell transplantation. Leukemia. 2013;27:836–42. https://doi.org/10.1038/leu.2012.319.
Uckelmann HJ, Armstrong SA. Chromatin complexes maintain self-renewal of myeloid progenitors in AML: opportunities for therapeutic intervention. Stem Cell Rep. 2020;15:6–12. https://doi.org/10.1016/j.stemcr.2020.05.013.
• Bernt KM, Zhu N, Sinha AU, Vempati S, Faber J, Krivtsov AV, et al. MLL-rearranged leukemia is dependent on aberrant H3K79 methylation by DOT1L. Cancer Cell. 2011;20:L66–78. https://doi.org/10.1016/j.ccr.2011.06.010. Demonstration of the dependence of MLLr AML on DOT1L.
• Daigle SR, Olhava EJ, Therkelsen CA, Majer CR, Sneeringer CJ, Song J, et al. Selective killing of mixed lineage leukemia cells by a potent small-molecule DOT1L inhibitor. Cancer Cell. 2011;20:53–65. https://doi.org/10.1016/j.ccr.2011.06.009. Pre-clinical development of a DOT1L inhibitor.
Meyer C, Burmeister T, Gröger D, Tsaur G, Fechina L, Renneville A, et al. The MLL recombinome of acute leukemias in 2017. Leukemia. 2018;32:273–84. https://doi.org/10.1038/leu.2017.213.
Daigle SR, Olhava EJ, Therkelsen CA, Basavapathruni A, Jin L, Boriack-Sjodin PA, et al. Potent inhibition of DOT1L as treatment of MLL-fusion leukemia. Blood. 2013;122:1017–25. https://doi.org/10.1182/blood-2013-04-497644.
•• Stein EM, Garcia-Manero G, Rizzieri DA, Tibes R, Berdeja JG, Savona MR, et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood. 2018;131:2661–9. https://doi.org/10.1182/blood-2017-09-806679. Initial phase 1 study of pinometostat in acute leukemia.
Shukla N, Wetmore C, O’Brien MM, Silverman LB, Brown P, Cooper TM, et al. Final report of phase 1 study of the DOT1L inhibitor, pinometostat (EPZ-5676), in children with relapsed or refractory MLL-r acute leukemia. Blood. 2016;128:2780. https://doi.org/10.1182/blood.V128.22.2780.2780.
• Goroschuk O, Kolosenko I, Vidarsdottir L, Azimi A, Palm-Apergi C. Polo-like kinases and acute leukemia. Oncogene. 2019;38:1–16. https://doi.org/10.1038/s41388-018-0443-5. An excellent review on the role of polo-like kinases in leukemia.
Liu X. Targeting polo-like kinases: a promising therapeutic approach for cancer treatment. Transl Oncol. 2015;8:185–95. https://doi.org/10.1016/j.tranon.2015.03.010.
Renner AG, Santos CD, Recher C, Bailly C, Créancier L, Kruczynski A, et al. Polo-like kinase 1 is overexpressed in acute myeloid leukemia and its inhibition preferentially targets the proliferation of leukemic cells. Blood. 2009;114:659–62. https://doi.org/10.1182/blood-208-12-195867.
Tao YF, Li ZH, Du WW, Xu LX, Ren JL, Li XL, et al. Inhibiting PLK1 induces autophagy of acute myeloid leukemia cells via mammalian target of rapamycin pathway dephosphorylation. Oncol Rep. 2017;37:1419–29. https://doi.org/10.3892/or.2017.5417.
Ikezoe T, Yang J, Nishioka C, Takezaki Y, Tasaka T, Togitani K, et al. A novel treatment strategy targeting polo-like kinase 1 in hematological malignancies. Leukemia. 2009;29:1564–76. https://doi.org/10.1038/leu.2009.94.
Steegmaier M, Hoffmann M, Baum A, Lénárt P, Petronczki M, Krssák M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Curr Biol. 2007;17:316–22. https://doi.org/10.1016/j.cub.2006.12.037.
• Moison C, Lavallée P, Thiollier C, Lehnertz B, Boivin I, Mayotte N, et al. Complex karyotype AML displays G2/M signature and hypersensitivity to PLK1 inhibition. Blood Adv. 2019;3:552–63. https://doi.org/10.1182/bloodadvances.2018.028480. Evidence for a PLK1 dependence in complex karyotype AML.
Simonetti G, Padella A, do Valle IF, Fontana MC, Fonzi E, Bruno S, et al. Aneuploid acute myeloid leukemia exhibits a signature of genomic alterations in the cell cycle and protein degradation machinery. Cancer. 2019;126:712–25. https://doi.org/10.1002/cncr.31837.
Dill V, Kauschinger J, Hauch RT, Buschhorn L, Odinius TO, Mȕller-Thomas C, et al. Inhibition of PLK1 by capped-dose volasertib exerts substantial efficacy in MDS and sAML while sparing healthy haematopoiesis. Eur J Haematol. 2020;104:125–37. https://doi.org/10.1111/ejh.13354.
•• Müller-Tidow C, Bug G, Lübbert M, Krämer A, Krauter J, Valent P, et al. A randomized, open-label, phase I/II trial to investigate the maximum tolerated dose of the polo-like kinase inhibitor BI 2536 in elderly patients with refractory/relapsed acute myeloid leukaemia. Br J Haematol. 2013;163:214–22. https://doi.org/10.1111/bjh.12518. The first study of a PLK inhibitor in AML.
Murphy T, Leber B, Bray MR, Chan SM, Gupta V, Khalaf D, et al. Preliminary results from a phase 1 study of Cfi-400495, a PLK4 inhibitor, in patients with acute myeloid leukemia and high risk MDS. Blood. 2020;136(Supplement 1):1–2. https://doi.org/10.1182/blood-2020-138822.
Jonas BA, Bixby DL, Brandwein JM, Yee KWL, Murphy T, Minden MD, et al. A phase 2 open-label, multicenter, dose optimization clinical study of the safety, tolerability, and pharmacokinetic (PK) and pharmacodynamic (PD) profiles of Cfi-400945 as a single agent or in combination with azacitidine or decitabine in patients with acute myeloid leukemia. Blood. 2020;136(Supplement 1):24–5. https://doi.org/10.1182/blood-2020-136004.
•• Zeidan AM, Ridinger M, Lin TL, Becker PS, Schiller GJ, Patel PA, et al. A phase Ib study of onvansertib, a novel oral PLK1 inhibitor, in combination therapy for patients with relapsed or refractory acute myeloid leukemia. Clin Cancer Res. 2020;26:6132–40. https://doi.org/10.1158/1078-0432.CCR-20-2586 Clinical trial of onvansertib in AML.
Zeidan AM, Lin T, Becker PS, Schiller GJ, Patel PA, Spira AI, et al. Updated analysis of a phase 1b/2 study of onvansertib, a PLK1 inhibitor, in combination with decitabine in patients with relapsed or refractory acute myeloid leukemia. Am Soc Hematol 2020 Annual Meeting: Abstract 1954.
Navada SC, Garcia-Manero G, Odchimar Reissig R, Pemmaraju N, Alvarado Y, Ohanian MN, et al. Rigosertib in combination with azacitidine in patients with myelodysplastic syndromes or acute myeloid leukemia: results of a phase 1 study. Leuk Res. 2020;94:106369. https://doi.org/10.1016/j.leukres.2020.106369.
Navada SC, Fruchtman SM, Odchimar-Reissig R, Demakos EP, Petrone ME, Zbyszewski PS, et al. A phase 1/2 study of rigosertib in patients with myelodysplastic syndromes (MDS) and MDS progressed to acute myeloid leukemia. Leuk Res. 2018;64:10–6. https://doi.org/10.1016/j.leukres.2017.11.006.
Garcia-Manero G, Fenaux P, Al-Kali A, Baer MR, Sekeres MA, Roboz GJ, et al. Rigosertib versus best supportive care for patients with high-risk myelodysplastic syndromes after failure of hypomethylating drugs (ONTIME): a randomized, controlled, phase 3 trial. Lancet Oncol. 2016;17:496–508. https://doi.org/10.1016/S1470-2045(16)00009-7.
•• Ottmann OG, Müller-Tidow C, Krämer A, Schlenk RF, Lübbert M, Bug G, et al. Phase I dose-escalation trial investigating volasertib as monotherapy or in combination with cytarabine in patients with relapsed/refractory acute myeloid leukaemia. Br J Haematol. 2019;184:1018–21. https://doi.org/10.1111/bjh.15204. Phase 1 study of volasertib in r/r AML showing safety as monotherapy and when combined with LDAC.
Döhner H, Lübbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426–33. https://doi.org/10.1182/blood-2014-03-560557.
Döhner H, Symeonidis A, Sanz AM, Deeren D, Demeter J, Anagnostopoulos A, et al. Phase III randomized trial of volasertib plus low-dose cytarabine (LDAC) versus placebo plus LDAC in patients aged ≥65 years with previously untreated AML, ineligible for intensive therapy. Eur Hematol Assoc 2016 Annual Meeting. Abstract S501.
Cortes J, Podoltsev N, Kantarjian H, Borthakur G, Zeidan AM, Stahl M, et al. Phase 1 dose escalation trial of volasertib in combination with decitabine in patients with acute myeloid leukemia. Int J Hematol. 2021;113:92–9. https://doi.org/10.1007/s12185-020-02994-8.
Gjertsen BT, Schöffski P. Discovery and development of the polo-like kinase inhibitor volasertib in cancer therapy. Leukemia. 2015;29:11–9. https://doi.org/10.1038/leu.2014.222.
Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, et al. BI 6727, a polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15:3094–102. https://doi.org/10.1158/1078-0432.CCR-08-2445.
Ciceri P, Müller S, O’Mahony A, Fedorov O, Filippakopoulos P, Hunt JP, et al. Dual kinase-bromodomain inhibitors for rationally designed polypharmacology. Nat Chem Biol. 2014;10:305–12. https://doi.org/10.1038/nchembio.1471.
Schöffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, et al. A phase I, dose-escalation study of the novel polo-like kinase inhibitor volasertib (BI 9727) in patients with advanced solid tumours. Eur J Cancer. 2012;48:179–86. https://doi.org/10.1016/j.ejca.2011.11.001.
Stadler WM, Vaughn DJ, Sonpavde G, Vogelzang NJ, Tagawa ST, Petrylak DP, et al. An open-label, single-arm, phase 2 trial of the polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer. 2014;120:976–82. https://doi.org/10.1002/cncr.28519.
Lin CC, Su WC, Yen CJ, Hsu CH, Su WP, Yeh KH, et al. A phase I study of two dosing schedules of volasertib (BI 6727), an intravenous polo-like kinase inhibitor, in patients with advanced solid malignancies. Br J Cancer. 2014;110:2434–40. https://doi.org/10.1038/bjc.2014.195.
Benetatos L, Dasoula A, Hatzimichael E, Syed N, Voukelatou M, Dranitsaris G, et al. Polo-like kinase 2 (SNK/PLK2) is a novel epigenetically regulated gene in acute myeloid leukemia and myelodysplastic syndromes: genetic and epigenetic interactions. Ann Hematol. 2011;90:1037–45. https://doi.org/10.1007/s00277-011-1193-4.
Deng S, Wang H, Jia C, Zhu S, Chu X, Ma Q, et al. MicroRNA-146a induces linage-negative bone marrow cell apoptosis and senescence by targeting polo-like kinase 2 expression. Arterioscler Thromb Vasc Biol. 2017;37:280–90. https://doi.org/10.1161/ATVBAHA.116.308378.
Beria I, Bossi RT, Brasca MG, Caruso M, Ceccarelli W, Fachin G, et al. NMS-P937, a 4,5-dihydro-1H-pyrazolo[4,3-h] quinazoline derivative as potent and selective polo-like kinase 1 inhibitor. Bioorg Med Chem Lett. 2011;21:2969–74. https://doi.org/10.1016/j.bmcl.2011.03.054.
Valsasina B, Beria I, Alli C, Alzani R, Avanzi N, Ballinari D, et al. NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther. 2012;11:1006–16. https://doi.org/10.1158/1535-7613.MCT-11-0765.
•• Weiss GJ, Jameson G, Von Hoff DD, Valsasina B, Davite C, Giulio CD, et al. Phase I dose escalation study of NMS-1286937, an orally available polo-like kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Investig New Drugs. 2018;36:85–95. https://doi.org/10.1007/s10637-017-0491-7. First clinical trial of onvansertib, which has improved PLK1 specificity.
Athuluri-Divakar SK, Vasquez-Del Carpio R, Dutta K, Baker SJ, Consenza SC, et al. A small molecule RAS-mimetic disrupts RAS association with effector proteins to block signaling. Cell. 2016;165:643–55. https://doi.org/10.1016/j.cell.2016.03.045.
Duffy MJ, Synnott NC, O’Grady S, Crown J. Targeting p53 for the treatment of cancer. Seminars Cancer Biol 2020:30LS1044-579X(20)30160-7. https://doi.org/10.1016/j.semcancer.2020.07.005.
Wong TN, Ramsingh R, Young AL, Miller CA, Touma W, Welch JS, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2014;518:552–5. https://doi.org/10.1038/nature13968.
Grinfeld J, Nangalia J, Baxter EJ, Wedge DC, Angelopoulos N, Cantrill R, et al. Classification and personalized prognosis in myeloproliferative neoplasms. N Engl J Med. 2018;379:1416–30. https://doi.org/10.1056/NEJMoa1716614.
Mrózek K, Eisfeld AK, Kohlschmidt J, Carroll AJ, Walker CJ, Nicolet D, et al. Complex karyotype in de novo acute myeloid leukemia: typical and atypical subtypes differ molecularly and clinically. Leukemia. 2019;33:1620–34. https://doi.org/10.1038/s41375-019-0390-3.
Rucker FG, Schlenk RF, Bullinger L, Kayser S, Teleanu V, Kett H, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–21. https://doi.org/10.1182/blood-2011-08-3715758.
Boettcher S, Miller PG, Sharma R, McConkey M, Leventhal M, Krivstov A, et al. A dominant-negative effect drives selection of TP53 missense mutations in myeloid malignancies. Science. 2019;365:599–604. https://doi.org/10.1126/science.aax3649.
Mims AS, Kohlschmidt J, Eisfeld AK, Mrózek K, Blachly JS, Orwick S, et al. Comparison of clinical and molecular characteristics of patients with acute myeloid leukemia and either TP73 or TP53 mutations. Leukemia. 2020. https://doi.org/10.1038/s41375-020-1007-6.
Prochazka KT, Pregartner G, Rücker FG, Heitzer E, Pabst G, Wölfer A, et al. Clinical implications of subclonal TP53 mutations in acute myeloid leukemia. Haematologica. 2019;104:516–23. https://doi.org/10.3324/haematol.2018.205013.
Welch JS, Pett AA, Miller CA, Fronick CC, O’Laughlin M, Fulton RS, et al. TP53 and decitabine in acute myeloid leukemia and myelodysplastic syndromes. N Engl J Med. 2016;375:2023–36. https://doi.org/10.1056/NEJMoa1605949.
Becker H, Pfeifer D, Ihorst G, Pantic M, Wehrle J, Rüter BH, et al. Monosomal karyotype and chromosome 17p loss or TP53 mutations in decitabine-treated patients with acute myeloid leukemia. Ann Hematol. 2020;99:1551–60. https://doi.org/10.1007/s00277-020-04082-7.
Short NJ, Kantarjian HM, Loghavi S, Huang X, Qiao W, Borhakur G, et al. Treatment with a 5-day versus a 10-day schedule of decitabine in older patients with newly diagnosed acute myeloid leukaemia: a randomized phase 2 trial. Lancet Haematol. 2019;6:e29–37. https://doi.org/10.1016/S2352-2036(1)30182-0.
Lambert JMR, Gorzov P, Veprintsev DB, Söderqvist SD, Bergman J, et al. PRIMA-1 reactivates mutant p53 by covalent binding to the core domain. Cancer Cell. 2009;15:376–88. https://doi.org/10.1016/j.ccr.2009.03.003.
• Zhang Q, Bykov VJN, Wiman KG, Zawacka-Pankau J, et al. APR-246 reactivates mutant p53 by targeting cysteines 124 and 277. Cell Death Dis. 2018;9:439. https://doi.org/10.1038/s41419-018-0463-7. Description of the mechanism by which APR-246 converts mutated p53 to wild-type activity.
Tessoulin B, Descamps G, Moreau P, Maïga S, Lodé L, Godon C, et al. PRIMA-1met induces myeloma cell death independent of p53 by impairing the GSH/ROS balance. Blood. 2014;124:1626–36. https://doi.org/10.1182/blood-2014-01-548800.
Maslah N, Salomao N, Drevon L, Verger E, Partouche N, Ly P, et al. Synergistic effects of PRIMA-1Met (APR-246) and 5-azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia. Haematologica. 2020;105:L1539–51. https://doi.org/10.3324/haematol.2019.218453.
•• Lehman S, Bykov VJN, Ali D, Andrén O, Cherif H, Tidefelt U, et al. Targeting p53 in vivo: a first-in-human study with p53-targeting compound APR-246 in refractory hematologic malignancies and prostate cancer. J Clin Oncol. 2012;30:3633–9. https://doi.org/10.1200/JCO.2011.40.7783. First-in-man study of a mutant TP53 inhibitor.
•• Deneberg S, Cherif H, Lazarevic V, Andersson P-O, von Euler M, Juliusson G, et al. An open-label phase I dose-finding study of APR-246 in hematological malignancies. Blood Cancer J. 2016;6:e447. https://doi.org/10.1038/bcj.2016.60. Optimization of infusion protocol allowed for increased APR-246 MTD in hematologic malignancies.
•• Sallman DA, DeZern AE, Garcia-Manero G, Steensma DP, Roboz GJ, Sekeres MA, et al. Eprenetapopt (APR-246) and azacitidine in TP53-mutant myelodysplastic syndromes. J Clin Oncol. 2021. https://doi.org/10.1200/JCO.20.02341 Online ahead of print. Phase 1 study of APR-146 in TP53-mutated myeloid malignancies.
•• Cluzeau T, Sebert M, Rahmé R, Cuzzubo S, Lehmann-Che J, Madelaine I, et al. Eprenetapopt plus azacitidine in TP53-mutated myelodysplastic syndromes and acute myeloid leukemia: a phase II study by the Groupe Francophone des Myélodysplasies (GFM). J Clin Oncol. 2021. https://doi.org/10.1200/JCO.20.02342 Online ahead of print. Phase 2 study of APR-146 in TP53-mutated myeloid malignancies.
Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–8.
•• Andreeff M, Kelly KR, Yee K, Assouline S, Strair R, Popplewell L, et al. Results of the phase 1 trial of RG7112, a small-molecule MDM2 antagonist in leukemia. Clin Cancer Res. 2016;22:868–76. https://doi.org/10.1158/1078-0432.CCR-15-0481. First phase 1 study of a MDM2 inhibitor in leukemia.
Ray-Coquard I, Blay JY, Italiano A, Cesne AL, Penel N, Zhi J, et al. Effect of the MDM2 antagonist RG7112 on the p53 pathway in patients with MDM2-amplified, well-differentiated or dedifferentiated liposarcoma: an exploratory proof-of-mechanism study. Lancet Oncol. 2012;13:1133–40. https://doi.org/10.1016/S1470-2045(12)70474-6.
Ding Q, Zhang Z, Liu JJ, Jiang N, Zhang J, Ross TM, et al. Discovery of RG7388, a potent and selective p53-MDM2 inhibitor in clinical development. J Med Chem. 2013;56:5979–83. https://doi.org/10.1021/jm400487c.
Yee K, Papayannidis C, Vey N, Dickinson MJ, Kelly KR, Assouline S, et al. Murine double minute 2 inhibition alone or with cytarabine in acute myeloid leukemia: results from an idasanutlin phase 1/1b study. Leuk Res. 2021;100:106489. https://doi.org/10.1016/j.leukres.2020.106489.
Reis B, Jukofsky L, Chen G, Martinelli G, Zhong HSo WV, et al. Acute myeloid leukemia patients’ clinical response to idasanutlin (RG7388) is associated with pre-treatment MDM2 protein expression in leukemic blasts. Haematologica. 2016;101:e185–8. https://doi.org/10.3324/haematol.2015.139717.
Mascarenhas J, Lu M, Kosiorek H, Virtgaym E, Xia L, Sandy L, et al. Oral idasanutlin in patients with polycythemia vera. Blood. 2019;134:525–33. https://doi.org/10.1182/blood.2018893545.
Montesinos P, Beckermann BM, Catalani O, Esteve J, Gamel K, Konopleva MY, et al. MIRROS: a randomized, placebo-controlled, Phase III trial of cytarabine ± idasanutlin in relapsed or refractory acute myeloid leukemia. Future Oncol. 2020;16:807–15. https://doi.org/10.2217/fon-2020-0044.
Siu LL, Italiano A, Miller WH, Blay JY, Gietema JA, Bang YJ, et al. Phase 1 dose escalation, food effect, and biomarker study of RG7388, a more potent second-generation MDM2 antagonist, in patients (pts) with solid tumors. J Clin Oncol. 2014;32:2535. https://doi.org/10.1200/jco.2014.32.15_suppl.2535.
Nemunaitis J, Young A, Ejadi S, Miller W, Chen LC, Nichols G, et al. Effects of posaconazole (a strong CYP3A4 inhibitor), two new tablet formulations, and food on the pharmacokinetics of idasanutlin, an MDM2 antagonist, in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2018;81:529–37. https://doi.org/10.1007/s00280-018-3521-z.
Higgins B, Glenn K, Walz A, Tovar C, Filipovic Z, Hussain S, et al. Preclinical optimization of MDM2 antagonist scheduling for cancer treatment by using a model-based approach. Clin Cancer Res. 2014;20:3742–52. https://doi.org/10.1158/1078-0432.CCR-14-0460.
Daver NG, Garcia JS, Jonas BA, Kelly KR, Assouline S, Brandwein JM, et al. Updated results from the venetoclax (Ven) in combination with idasanutlin (Idasa) arm of a phase 1b trial in elderly patients with relapsed or refractory (R/R) AML ineligible for cytotoxic chemotherapy. Blood. 2019;134:229.
Rew Y, Sun D. Discovery of a small molecule MDM2 inhibitor (AMG 232) for treating cancer. J Med Chem. 2014;57:6332–41. https://doi.org/10.1021/jm500627s.
Gluck WL, Gounder MM, Frank R, Eskens F, Blay JY, Cassier PA, et al. Phase 1 study of the MDM2 inhibitor AMG 232 in patients with advanced p53 wild-type solid tumors or multiple myeloma. Investig New Drugs. 2020;38:831–43. https://doi.org/10.1007/s10637-019-00840-1.
•• Erba HP, Becker PS, Shami PJ, Grunwald MR, Flesher DL, Zhu M, et al. Phase 1b study of the MDM2 inhibitor AMG 232 with or without trametinib in relapsed/refractory acute myeloid leukemia. Blood Adv. 2019;3:1393–949. https://doi.org/10.1182/bloodadvances.2019030916. Phase 1 study showing safety and evidence for activity of AMG-232 in AML.
Carvajal LA, Neriah DB, Senecal A, Benard L, Thiruthuvanathan V, Yatsenko T, et al. Dual inhibition of MDMX and MDM2 as a therapeutic strategy in leukemia. Sci Transl Med. 2018;10:eaao3003. https://doi.org/10.1126/scitranslmed.aao3003.
Sallman DA, Borate U, Cull EH, Donnellan WB, Komrokji RS, Steidl UG, et al. Phase 1/1b study of the stapled peptide ALRN-6924, a dual inhibitor of MDMX and MDM2, as monotherapy or in combination with cytarabine for the treatment of relapsed/refractory AML and advanced MDS with TP53 wild-type. Blood. 2018;132:4066. https://doi.org/10.1182/blood-2018-99-118780.
Armstrong SA, Staunton JE, Silverman LB, Pieters R, den Boer ML, Minden MD, et al. MLL translocations specify a distinct gene expression profile that distinguishes a unique leukemia. Nat Genet. 30:41–7. https://doi.org/10.1038/ng765.
Hinai ASAA, Pratcorona M, Grob T, Kavelaars FG, Bussaglia E, Sanders MA, et al. The landscape of KMT2A-PTD AML: concurrent mutations, gene expression signatures, and clinical outcome. Hemasphere. 2019;3:e181. https://doi.org/10.1097/HS9.0000000000000181.
Alcalay M, Tiacci E, Bergomas R, Bigerna B, Venturini E, Minardi SP, et al. Acute myeloid leukemia bearing cytoplasmic nucleophosmin (NPMc+ AML) shows a distinct gene expression profile characterized by up-regulation of genes involved in stem-cell maintenance. Blood. 2005;106:899–902. https://doi.org/10.1182/blood-2005-02-0560.
Mullighan CG, Kennedy A, Zhou X, Radtke I, Phillips LA, Shurtleff SA, et al. Pediatric acute myeloid leukemia with NPM1 mutations is characterized by a gene expression profile with dysregulated HOX gene expression distinct from MLL-rearranged leukemias. Leukemia. 2007;9:2000–9. https://doi.org/10.1038/sj.leu.2404808.
Spencer DH, Young MA, Lamprecht TL, Helton NM, Fulton R, O'Laughlin M, et al. Epigenomic analysis of the HOX gene loci reveals mechanisms that may control canonical expression patterns in AML and normal hematopoietic cells. Leukemia. 2015;6:1279–89. https://doi.org/10.1038/leu.2015.6.
Caligiuri MA, Strout MP, Lawrence D, Arthur DC, Baer MR, Yu F, et al. Rearrangement of ALL1 (MLL) in acute myeloid leukemia with normal cytogenetics. Cancer Res. 1998;58:55–9.
Döhner K, Tobis K, Ulrich R, Fröhling S, Benner A, Schlenk RF, et al. Prognostic significance of partial tandem duplications of the MLL gene in adult patients 16 to 60 years old with acute myeloid leukemia and normal cytogenetics: a study of the Acute Myeloid Leukemia Study Group Ulm. J Clin Oncol. 2002;20:3254–61. https://doi.org/10.1200/JCO.2002.09.088.
Falini B, Brunetti L, Sportoletti P, Martelli MP. NPM1-mutated acute myeloid leukemia: from bench to bedside. Blood. 2020;136:1707–21. https://doi.org/10.1182/blood.2019004226.
Brown P, McIntyre E, Rau R, Meshinchi S, Lacayo N, Dahl G, et al. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood. 2007;110:979–85. https://doi.org/10.1182/blood-2007-02-076604.
Bhatlekar S, Fields JZ, Boman BM. Role of HOX genes in stem cell differentiation and cancer. Stem Cells Int. 2018;2018:3569493. https://doi.org/10.1155/2018/3569493.
Alharbi RA, Pettengell R, Pandha HS, Morgan R. The role of HOX genes in normal hematopoiesis and acute leukemia. Leukemia. 2013;27:1000–8. https://doi.org/10.1038/leu.2012.356.
Antunes ETB, Ottersbach K. The MLL/SET family and haematopoiesis. Biochim Biophys Acta Gene Regul Mech. 1863;2020:194579. https://doi.org/10.1016/j.bbagrm.2020.194579.
Milne TA, Briggs SD, Brock HW, Martin ME, Gibbs D, Allis CD, et al. MLL targets SET domain methyltransferase activity to Hox gene promoters. Mol Cell. 2002;10:1107–17. https://doi.org/10.1016/s1097-2765(02)00741-4.
Dorrance AM, Liu S, Yuan W, Becknell B, Arnoczky KJ, Guimond M, et al. Mll partial tandem duplication induces aberrant Hox expression in vivo via specific epigenetic alterations. J Clin Invest. 2006;116:2707–16. https://doi.org/10.1172/JCI25546.
Yokoyama A, Wang Z, Wysocka J, Sanyal M, Aufiero DJ, Kitabayashi I, et al. Leukemia proto-oncoprotein MLL forms a SET1-like histone methyltransferase complex with menin to regulate Hox gene expression. Mol Cell Biol. 2004;24:5639–49. https://doi.org/10.1128/MCB.24.13.5639-5649.2004.
Yokoyama A, Somervaille TC, Smith KS, Rozenblatt-Rosen O, Meyerson M, Cleary ML. The menin tumor suppressor protein is an essential oncogenic cofactor for MLL-associated leukemogenesis. Cell. 2005;123:207–18. https://doi.org/10.1016/j.cell.2005.09.025.
Yokoyama A, Cleary ML. Menin critically links MLL proteins with LEDGF on cancer-associated target genes. Cancer Cell. 2008;14:36–46. https://doi.org/10.1016/j.ccr.2008.05.003.
Chen YX, Yan J, Keeshan K, Tubbs AT, Wang H, Silva A, et al. The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression. Proc Natl Acad Sci U S A. 2006;103:1018–23. https://doi.org/10.1073/pnas.0510347103.
Vassiliou GS, Cooper JL, Rad R, Li J, Rice S, Uren A, et al. Mutant nucleophosmin and cooperating pathways drive leukemia initiation and progression in mice. Nat Genet. 2011;43:470–5. https://doi.org/10.1038/ng.796.
Dovey OM, Cooper JL, Mupo A, Grove CS, Lynn C, Conte N, et al. Molecular synergy underlies the co-occurrence patterns and phenotype of NPM1-mutant acute myeloid leukemia. Blood. 2017;130:1911–22. https://doi.org/10.1182/blood-2017-01-760595.
Uckelmann HJ, Kim SM, Wong EM, Hatton C, Giovinazzo H, Gadrey JY, et al. Therapeutic targeting of preleukemia cells in a mouse model of NPM1 mutant acute myeloid leukemia. Science. 2020;367:586–90. https://doi.org/10.1126/science.aax5863.
Brunetti L, Gundry MC, Sorcini D, Guzman AG, Huang YH, Ramabadran R, et al. Mutant NPM1 maintains the leukemic state through HOX expression. Cancer Cell. 2018;34:499–512. https://doi.org/10.1016/j.ccell.2018.08.005.
Gu X, Ebrahem Q, Mahfouz RZ, Hasipek M, Enane F, Radivoyevitch T, et al. Leukemogenic nucleophosmin mutation disrupts the transcription factor hub that regulates granulomonocytic fates. J Clin Invest. 2018;128:4260–79. https://doi.org/10.1172/JCI97117.
Kühn MW, Song E, Feng Z, Sinha A, Chen CW, Deshpande AJ, et al. Targeting chromatin regulators inhibits leukemogenic gene expression in NPM1 mutant leukemia. Cancer Discov. 2016;6:1166–81. https://doi.org/10.1158/2159-8290.CD-16-0237.
Cierpicki T, Grembecka J. Challenges and opportunities in targeting the menin-MLL interaction. Future Med Chem. 2014;6:447–62. https://doi.org/10.4155/fmc.13.214.
• Grembecka J, He S, Shi A, Purohit T, Muntean AG, Sorenson RJ, et al. Menin-MLL inhibitors reverse oncogenic activity of MLL fusion proteins in leukemia. Nat Chem Biol. 2012;8:277–84. https://doi.org/10.1038/nchembio.773. First pre-clinical report of a menin inhibitor.
Perner F, Armstrong SA. Targeting chromatin complexes in myeloid malignancies and beyond: from basic mechanisms to clinical innovation. Cells. 2020;9:2721. https://doi.org/10.3390/cells9122721.
He S, Malik B, Borkin D, Miao H, Shukla S, Kempinska K, et al. Menin-MLL inhibitors block oncogenic transformation by MLL-fusion proteins in a fusion partner-independent manner. Leukemia. 2016;30:508–13. https://doi.org/10.1038/leu.2015.144.
Timmers HTM, Özyerli Göknar E, Nizamuddin S. A box of chemistry to inhibit the MEN1 tumor suppressor gene promoting leukemia. Chem Med Chem. 2021. https://doi.org/10.1002/cmdc.202000972 Online ahead of print.
•• Klossowski S, Miao H, Kempinska K, Wu T, Purohit T, Kim E, et al. Menin inhibitor MI-3454 induces remission in MLL1-rearranged and NPM1-mutated models of leukemia. J Clin Invest. 2020;130:981–97. https://doi.org/10.1172/JCI129126. Report of an optimized menin inhibitor suitable for in vivo use.
Kessler L, Wu T, Grembecka J, Cierpicki T, Purohit T, Miaoand H, et al. Discovery of novel menin-MLL small molecule inhibitors that display high potency and selectivity in vitro and in vivo. Eur J Cancer. 2016;69S:88. https://doi.org/10.1016/S0959-8049(16)32859-3.
Wu T, Kessler L, Li S, Purohit T, Li S, Miao H, et al. A novel small molecule menin-MLL inhibitor for potential treatment of MLL-rearranged leukemias. Cancer Res. 2017;77(13 Suppl):5077. https://doi.org/10.1158/1538-7445.AM2017-5077.
Burrows F, Wu T, Kessler L, Li S, Zhang J, Zarrinkar P, et al. A novel small molecule menin-MLL inhibitor for potential treatment of MLL-rearranged leukemias and NPM1/DNMT3A-mutant AML. Mol Cancer Ther. 2018;17(1 Suppl):LB-A27. https://doi.org/10.1158/1535-7163.TARG-17-LB-A27.
•• Wang ES, Altman JK, Pettit K, De Botton S, Walter RP, Fenaux P, et al. Preliminary data on a phase 1/2A first in human study of the menin-KMT2A (MLL) inhibitor KO-539 in patients with relapsed or refractory acute myeloid leukemia. Blood. 2020;136(Supplement 1):7–8. https://doi.org/10.1182/blood-2020-134942. First-in-man report of the menin inhibitor KO-539.
Libbrecht C, Xie HM, Kingsley MC, Haladyna JN, Riedel SS, Alikarami F, et al. Menin is necessary for long term maintenance of meningioma-1 driven leukemia. Leukemia. 2021. https://doi.org/10.1038/s41375-021-01146-z Online ahead of print.
Dzama MM, Steiner M, Rausch J, Sasca D, Schönfeld J, Kunz K, et al. Synergistic targeting of FLT3 mutations in AML via combined menin-MLL and FLT3 inhibition. Blood. 2020;136:2442–56. https://doi.org/10.1182/blood.2020005037.
Schmidt L, Heyes E, Scheiblecker L, Eder T, Volpe G, Frampton J, et al. CEBPA-mutated leukemia is sensitive to genetic and pharmacological targeting of the MLL1 complex. Leukemia. 2019;33:1608–19. https://doi.org/10.1038/s41375-019-0382-3.
•• Krivtsov AV, Evans K, Gadrey JY, Eschle BK, Hatton C, Uckelmann HJ, et al. A Menin-MLL inhibitor induces specific chromatin changes and eradicates disease in models of MLL-rearranged leukemia. Cancer Cell. 2019;36:660–73. https://doi.org/10.1016/j.ccell.2019.11.001. Report of a second, structurally distinct menin inhibitor.
•• McGeehan G. A first-in-class menin-MLL1 antagonist for the treatment of MLL-r and NPM1 mutant leukemias. Presentation at the 2020 American Association for Cancer Research Virtual Annual Meeting I 2020. First report of a menin inhibitor clinical trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Human and Animal Rights and Informed Consent
This article contains no studies with human or animal subjects performed by either of the authors.
Conflict of Interest
Dr. Nicole Grieselhuber declares that she has no conflict of interest.
Dr. Alice Mims has served on the advisory boards for Syndax Pharmaceuticals, Kura Oncology, Jazz Pharmaceuticals, and AbbVie Pharmaceuticals.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Acute Myeloid Leukemias
Rights and permissions
About this article
Cite this article
Grieselhuber, N.R., Mims, A.S. Novel Targeted Therapeutics in Acute Myeloid Leukemia: an Embarrassment of Riches. Curr Hematol Malig Rep 16, 192–206 (2021). https://doi.org/10.1007/s11899-021-00621-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11899-021-00621-9