Summary
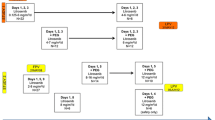
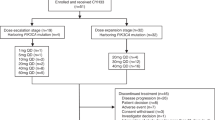
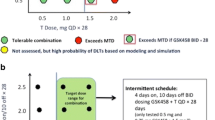
Background Pharmacological inhibition of polo-like kinase 1 (PLK1) represents a new approach for the treatment of solid tumors. This study was aimed at determining the first cycle dose-limiting toxicities (DLTs) and related maximum tolerated dose (MTD) of NMS-1286937, a selective ATP-competitive PLK1-specific inhibitor. Secondary objectives included evaluation of its safety and pharmacokinetic (PK) profile in plasma, its antitumor activity, and its ability to modulate intracellular targets in biopsied tissue. Methods This was a Phase I, open-label, dose-escalation trial in patients with advanced/metastatic solid tumors. A treatment cycle comprised 5 days of oral administration followed by 16 days of rest, for a total of 21 days (3-week cycle). Results Nineteen of 21 enrolled patients with confirmed metastatic disease received study medication. No DLTs occurred at the first 3 dose levels (6, 12, and 24 mg/m2/day). At the subsequent dose level (48 mg/m2/day), 2 of 3 patients developed DLTs. An intermediate level of 36 mg/m2/day was therefore investigated. Four patients were treated and two DLTs were observed. After further cohort expansion, the MTD and recommended phase II dose (RP2D) were determined to be 24 mg/m2/day. Disease stabilization, observed in several patients, was the best treatment response observed. Hematological toxicity (mostly thrombocytopenia and neutropenia) was the major DLT. Systemic exposure to NMS-1286937 increased with dose and was comparable between two cycles of treatment following oral administration of the drug. Conclusions This study successfully identified the MTD and DLTs for NMS-1286937 and characterized its safety profile.
Similar content being viewed by others
References
Sunkel CE, Glover DM (1988) polo, a mitotic mutant of Drosophila displaying abnormal spindle poles. J Cell Sci 89(Pt 1):25–38
Liu X, Erikson RL (2003) Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci U S A 100(10):5789–5794. doi:10.1073/pnas.1031523100
de Carcer G, Manning G, Malumbres M (2011) From Plk1 to Plk5: functional evolution of polo-like kinases. Cell Cycle (Georgetown, Tex) 10(14):2255–2262. doi:10.4161/cc.10.14.16494
Barr FA, Sillje HH, Nigg EA (2004) Polo-like kinases and the orchestration of cell division. Nat Rev Mol Cell Biol 5(6):429–440. doi:10.1038/nrm1401
Feng YB, Lin DC, Shi ZZ, Wang XC, Shen XM, Zhang Y, Du XL, Luo ML, Xu X, Han YL, Cai Y, Zhang ZQ, Zhan QM, Wang MR (2009) Overexpression of PLK1 is associated with poor survival by inhibiting apoptosis via enhancement of survivin level in esophageal squamous cell carcinoma. Int J Cancer 124(3):578–588. doi:10.1002/ijc.23990
Otsu H, Iimori M, Ando K, Saeki H, Aishima S, Oda Y, Morita M, Matsuo K, Kitao H, Oki E, Maehara Y (2016) Gastric cancer patients with High PLK1 expression and DNA aneuploidy correlate with poor prognosis. Oncology 91(1):31–40. doi:10.1159/000445952
Weichert W, Denkert C, Schmidt M, Gekeler V, Wolf G, Kobel M, Dietel M, Hauptmann S (2004) Polo-like kinase isoform expression is a prognostic factor in ovarian carcinoma. Br J Cancer 90(4):815–821. doi:10.1038/sj.bjc.6601610
Weichert W, Kristiansen G, Winzer KJ, Schmidt M, Gekeler V, Noske A, Muller BM, Niesporek S, Dietel M, Denkert C (2005) Polo-like kinase isoforms in breast cancer: expression patterns and prognostic implications. Virchows Archiv : An Intern J Pathol 446(4):442–450. doi:10.1007/s00428-005-1212-8
Weichert W, Kristiansen G, Schmidt M, Gekeler V, Noske A, Niesporek S, Dietel M, Denkert C (2005) Polo-like kinase 1 expression is a prognostic factor in human colon cancer. World J Gastroenterol 11(36):5644–5650
Knecht R, Elez R, Oechler M, Solbach C, von Ilberg C, Strebhardt K (1999) Prognostic significance of polo-like kinase (PLK) expression in squamous cell carcinomas of the head and neck. Cancer Res 59(12):2794–2797
Eckerdt F, Yuan J, Strebhardt K (2005) Polo-like kinases and oncogenesis. Oncogene 24(2):267–276. doi:10.1038/sj.onc.1208273
Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, Staubli U, Bereiter-Hahn J, Strebhardt K, Kuhl D (1999) The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J 18(20):5528–5539. doi:10.1093/emboj/18.20.5528
Hyun SY, Hwang HI, Jang YJ (2014) Polo-like kinase-1 in DNA damage response. BMB Rep 47(5):249–255
Wu J, Ivanov AI, Fisher PB, Fu Z (2016) Polo-like kinase 1 induces epithelial-to-mesenchymal transition and promotes epithelial cell motility by activating CRAF/ERK signaling. elife 5. doi:10.7554/eLife.10734
Strebhardt K (2015) Drugging Plk1: An attractive approach to inhibit androgen receptor signaling. Cell cycle (Georgetown, Tex) 14(14):2193–2194. doi:10.1080/15384101.2015.1056611
Zhang Z, Hou X, Shao C, Li J, Cheng JX, Kuang S, Ahmad N, Ratliff T, Liu X (2014) Plk1 inhibition enhances the efficacy of androgen signaling blockade in castration-resistant prostate cancer. Cancer Res 74(22):6635–6647. doi:10.1158/0008-5472.can-14-1916
Zhang Z, Chen L, Wang H, Ahmad N, Liu X (2015) Inhibition of Plk1 represses androgen signaling pathway in castration-resistant prostate cancer. Cell Cycle (Georgetown, Tex) 14(13):2142–2148. doi:10.1080/15384101.2015.1041689
Thoma C (2014) Prostate cancer: PLK-1 inhibition improves abiraterone efficacy. Nat Rev Urol 11(11):603. doi:10.1038/nrurol.2014.287
Xiao D, Yue M, Su H, Ren P, Jiang J, Li F, Hu Y, Du H, Liu H, Qing G (2016) Polo-like Kinase-1 Regulates Myc Stabilization and Activates a Feedforward Circuit Promoting Tumor Cell Survival. Mol Cell 64(3):493–506. doi:10.1016/j.molcel.2016.09.016
Luo J, Emanuele MJ, Li D, Creighton CJ, Schlabach MR, Westbrook TF, Wong KK, Elledge SJ (2009) A genome-wide RNAi screen identifies multiple synthetic lethal interactions with the Ras oncogene. Cell 137(5):835–848. doi:10.1016/j.cell.2009.05.006
Wang J, Hu K, Guo J, Cheng F, Lv J, Jiang W, Lu W, Liu J, Pang X, Liu M (2016) Suppression of KRas-mutant cancer through the combined inhibition of KRAS with PLK1 and ROCK. Nat Commun 7:11363. doi:10.1038/ncomms11363
Smith MR, Wilson ML, Hamanaka R, Chase D, Kung H, Longo DL, Ferris DK (1997) Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem Biophys Res Commun 234(2):397–405
Spankuch-Schmitt B, Wolf G, Solbach C, Loibl S, Knecht R, Stegmuller M, von Minckwitz G, Kaufmann M, Strebhardt K (2002) Downregulation of human polo-like kinase activity by antisense oligonucleotides induces growth inhibition in cancer cells. Oncogene 21(20):3162–3171. doi:10.1038/sj.onc.1205412
Schoffski P (2009) Polo-like kinase (PLK) inhibitors in preclinical and early clinical development in oncology. Oncologist 14(6):559–570. doi:10.1634/theoncologist.2009-0010
Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, Haslinger C, Garin-Chesa P, Adolf GR (2009) BI 6727, A Polo-like Kinase Inhibitor with Improved Pharmacokinetic Profile and Broad Antitumor Activity. Clin Cancer Res 15(9):3094–3102. doi:10.1158/1078-0432.ccr-08-2445
Filippakopoulos P, Qi J, Picaud S, Shen Y, Smith WB, Fedorov O, Morse EM, Keates T, Hickman TT, Felletar I, Philpott M, Munro S, McKeown MR, Wang Y, Christie AL, West N, Cameron MJ, Schwartz B, Heightman TD, La Thangue N, French CA, Wiest O, Kung AL, Knapp S, Bradner JE (2010) Selective inhibition of BET bromodomains. Nature 468(7327):1067–1073. doi:10.1038/nature09504
Schoffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, Taton M, Fritsch H, Glomb P, Munzert G (2012) A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Europ J Cancer (Oxford, England: 1990) 48(2):179–186. doi:10.1016/j.ejca.2011.11.001
Dohner H, Lubbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, Lepretre S, Reman O, Turlure P, Ottmann OG, Muller-Tidow C, Kramer A, Raffoux E, Dohner K, Schlenk RF, Voss F, Taube T, Fritsch H, Maertens J (2014) Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood 124(9):1426–1433. doi:10.1182/blood-2014-03-560557
Valsasina B, Beria I, Alli C, Alzani R, Avanzi N, Ballinari D, Cappella P, Caruso M, Casolaro A, Ciavolella A, Cucchi U, De Ponti A, Felder E, Fiorentini F, Galvani A, Gianellini LM, Giorgini ML, Isacchi A, Lansen J, Pesenti E, Rizzi S, Rocchetti M, Sola F, Moll J (2012) NMS-P937, an orally available, specific small-molecule polo-like kinase 1 inhibitor with antitumor activity in solid and hematologic malignancies. Mol Cancer Ther 11(4):1006–1016. doi:10.1158/1535-7163.mct-11-0765
Beria I, Bossi RT, Brasca MG, Caruso M, Ceccarelli W, Fachin G, Fasolini M, Forte B, Fiorentini F, Pesenti E, Pezzetta D, Posteri H, Scolaro A, Re Depaolini S, Valsasina B (2011) NMS-P937, a 4,5-dihydro-1H-pyrazolo[4,3-h]quinazoline derivative as potent and selective Polo-like kinase 1 inhibitor. Bioorg Med Chem Lett 21(10):2969–2974. doi:10.1016/j.bmcl.2011.03.054
Hartsink-Segers SA, Exalto C, Allen M, Williamson D, Clifford SC, Horstmann M, Caron HN, Pieters R, Den Boer ML (2013) Inhibiting Polo-like kinase 1 causes growth reduction and apoptosis in pediatric acute lymphoblastic leukemia cells. Haematologica 98(10):1539–1546. doi:10.3324/haematol.2013.084434
Casolaro A, Golay J, Albanese C, Ceruti R, Patton V, Cribioli S, Pezzoni A, Losa M, Texido G, Giussani U, Marchesi F, Amboldi N, Valsasina B, Bungaro S, Cazzaniga G, Rambaldi A, Introna M, Pesenti E, Alzani R (2013) The Polo-Like Kinase 1 (PLK1) inhibitor NMS-P937 is effective in a new model of disseminated primary CD56+ acute monoblastic leukaemia. PLoS One 8(3):e58424. doi:10.1371/journal.pone.0058424
Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States. Natl Cancer Inst Canada J Natl Cancer Inst 92(3):205–216
Cucchi U, Gianellini LM, De Ponti A, Sola F, Alzani R, Patton V, Pezzoni A, Troiani S, Saccardo MB, Rizzi S, Giorgini ML, Cappella P, Beria I, Valsasina B (2010) Phosphorylation of TCTP as a marker for polo-like kinase-1 activity in vivo. Anticancer Res 30(12):4973–4985
Simon R, Freidlin B, Rubinstein L, Arbuck SG, Collins J, Christian MC (1997) Accelerated titration designs for phase I clinical trials in oncology. J Natl Cancer Inst 89(15):1138–1147
Addington J, Freimer M (2016) Chemotherapy-induced peripheral neuropathy: an update on the current understanding. F1000Res 5. doi:10.12688/f1000research.8053.1
Ackermann S, Goeser F, Schulte JH, Schramm A, Ehemann V, Hero B, Eggert A, Berthold F, Fischer M (2011) Polo-like kinase 1 is a therapeutic target in high-risk neuroblastoma. Clin Cancer Res 17(4):731–741. doi:10.1158/1078-0432.CCR-10-1129
Grinshtein N, Datti A, Fujitani M, Uehling D, Prakesch M, Isaac M, Irwin MS, Wrana JL, Al-Awar R, Kaplan DR (2011) Small molecule kinase inhibitor screen identifies polo-like kinase 1 as a target for neuroblastoma tumor-initiating cells. Cancer Res 71(4):1385–1395. doi:10.1158/0008-5472.CAN-10-2484
Acknowledgments
We thank the cancer patients who willingly agreed to participate in the study. We also acknowledge the excellent contribution of Mariangela Mariani (Study director), Luisella Bonomini (Clinical project Manager), Benedetta Reinach (Clinical Data Manager), Manuela Pesenti, Marina Ripamonti and Patrizia Crivori of CLIOSS S.r.l for their professional support in providing information and documentation and Sonia Colombini (Programmer) of CLIOSS S.r.l for her support in programming. Alessia Casolaro and Roberta Ceruti of NMS for assistance with biopsy analyses, Cinzia Pellizzoni for PK analysis support. Professional medical writing assistance during the preparation of this manuscript was provided by Prasad Kulkarni, Ph.D., CMPP of Asclepius Medical Communications LLC, Ridgewood, New Jersey, USA and was funded by the Cancer Treatment Centers of America.
Author information
Authors and Affiliations
Contributions
Conception and design: C Davite and D. Von Hoff.
Acquisition of data: G. Weiss, G. Jameson, F. Fiorentini, C. Di Giulio, and R. Ramanathan.
Analysis and interpretation of data: G. Weiss, B. Valsasina, R. Alzani, P Carpinelli, and F. Fiorentini.
Writing, review, and/or revision of the manuscript: G. Weiss, A. Isacchi, A. Galvani, B. Valsasina, C. Di Giulio, C. Davite, G. Jameson, R. Ramanathan, and D. Von Hoff.
Administrative, technical, or material support (eg, reporting or organizing data, constructing databases): C. Di Giulio and A. Di Sanzo.
Study supervision: G. Weiss.
Corresponding author
Ethics declarations
Conflict of interest
Glen Weiss has no direct conflicts of interest related to this work, but has the following unrelated disclosures: consulting or advisory roles with Pharmatech, Paradigm, Blend Therapeutics, and Viomics; service on speakers’ bureaus with Medscape, Novartis, and Merck; and receipt of travel and accommodation expenses from Nantworks. Rachele Alzani, Patrizia Carpinelli, Arturo Galvani, Antonella Isacchi, and Barbara Valsasina are employees of Nerviano Sciences s.r.l. Francesco Fiorentini is an employee of Accelera s.r.l. Cristina Davite, Claudia Di Giulio, and Alessandro Di Salvo are employees of Clioss s.r.l. Gayle Jameson, Daniel Von Hoff, and Ramesh Ramanathan have no conflicts to disclose.
Ethical approval
The study protocol, amendments to the protocol, and the sample informed consent file were reviewed and approved by the Institutional Review Board (IRB) at the study site. The study conformed to Good Clinical Practice guidelines published by the International Council for Harmonization (ICH), ethical principles as set forth in the Declaration of Helsinki (1996 version), and all applicable local regulatory requirements and laws.
Informed consent
Signed written informed consent was obtained from each patient prior to undertaking any trial-related procedure.
Funding
This study was funded by Nerviano Medical Sciences S.r.l, Nerviano, Italy.
Electronic supplementary material
ESM 1
(DOCX 26 kb)
Rights and permissions
About this article
Cite this article
Weiss, G.J., Jameson, G., Von Hoff, D.D. et al. Phase I dose escalation study of NMS-1286937, an orally available Polo-Like Kinase 1 inhibitor, in patients with advanced or metastatic solid tumors. Invest New Drugs 36, 85–95 (2018). https://doi.org/10.1007/s10637-017-0491-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10637-017-0491-7