Abstract
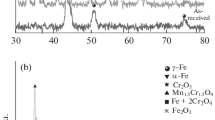
Serious pipe corrosion caused by accumulated \({\text{S}}^{{{2} - }}\) in Bayer solutions occurs when treating high-sulfur diasporic bauxite by the Bayer process at elevated temperatures. The effects of \({\text{S}}^{{{2} - }}\) and \({\text{S}}_{{2}} {\text{O}}_{{3}}^{{{2} - }}\) anions on the corrosion behavior of 16Mn low-alloy steel have been investigated under simulated high-temperature Bayer digestion conditions. The structure of the corrosion layers was analyzed. Results show that \({\text{S}}^{{{2} - }}\) anions markedly accelerate such corrosion because of the generation of loose FeS-containing corrosion product, whereas \({\text{S}}_{{2}} {\text{O}}_{{3}}^{{{2} - }}\) anions with concentration > 8.7 g L−1 can remarkably retard the corrosion process by forming a compact magnetite layer with dense and fine particles. Corrosion surface topography analyses support all the experimental corrosion results. Thus, \({\text{S}}_{{2}} {\text{O}}_{{3}}^{{{2} - }}\) represents an effective corrosion inhibitor for 16Mn low-alloy steel, and \({\text{S}}^{{{2} - }}\) to \({\text{S}}_{{2}} {\text{O}}_{{3}}^{{{2} - }}\) transformations may contribute to preventing equipment corrosion when using high-sulfur bauxite in the Bayer process.
Similar content being viewed by others
References
X. Li, C. Li, Q. Zhou, T. Qi, G. Liu, and Z. Peng, Int. J. Miner. Process. 137, 9. (2015).
W. Chai, Y. Huang, W. Peng, G. Han, Y. Cao, and J. Liu, Miner. Eng. 129, 93. (2018).
X. Hu, W. Chen, and Q. Xie, Trans. Nonferrous Metal Soc. 21, 1641. (2011).
B. Quan, J. Li, and C. Chen, Mater. Res. Express. 7, 035602. (2020).
Z. Liu, H. Yan, W. Ma, and P. Xiong, Mining Metall. Explor. 37, 1617. (2020).
L. Freire, M.J. Carmezim, M.G.S. Ferreira, and M.F. Montemor, Electrochim. Acta. 56, 5280. (2011).
I. Betova, M. Bojinov, O. Hyökyvirta, and T. Saario, Corros. Sci. 52, 1499. (2010).
D. Singbeil, and D. Tromans, J. Electrochem. Soc. 128, 2065. (1981).
D.C. Crowe, and D. Tromans, Corrosion 44, 142. (Houston, TX U. S.) (1988).
P.M. Singh, O. Ige, and J. Mahmood, Corrosion 59, 843. (Houston, TX U. S.) (2003).
R. Feng, J. Beck, M. Ziomek-Moroz, and S.N. Lvov, Electrochim. Acta. 241, 341. (2017).
R. Sriram, and D. Tromans, Corros. Sci. 25, 79. (1985).
Q. Xie, and W. Chen, Corros. Sci. 86, 252. (2014).
H. Fu, C. Chen, J. Li, Y. Lan, L. Wang, and J. Yuan, Mater. Res. Express. 6, 1065a9. (2019).
B.L. Quan, J.Q. Li, and C.Y. Chen, Int. J. Corros. 2016, 1. (2016).
B.L. Quan, J.Q. Li, and C.Y. Chen, Mater. Res. Express. 6, 10. (2019).
J. Yuan, C. Chen, J. Li, B. Quan, L. Wang, Y. Lan, X. Du, and H. Fu, Mater. Express. 9, 914. (2019).
J. Yuan, C. Chen, J. Li, B. Quan, Y. Lan, L. Wang, H. Fu, and J. Gai, Metals. 10, 1283. (2020).
Q.L. Xie, W.M. Chen, and Q. Yang, Corrosion 70, 842. (Houston, TX , U. S.) (2014).
X. Li, C. Li, Z. Peng, G. Liu, Q. Zhou, and T. Qi, Trans. Nonferrous Met. Soc. 25, 608. (2015).
X. Li, F. Niu, G. Liu, T. Qi, Q. Zhou, and Z. Peng, Trans. Nonferrous Met. Soc. 27, 908. (2017).
F. Hong, L. Feng, W. Qiao, and X. Lu, Heat Treat. Met. 44, 165. (Beijing, China) (2019).
H.L. Watts, and D.W. Utley, Anal. Chem. 25, 864. (1953).
X. Li, Z. Zhou, Y. Wang, Q. Zhou, T. Qi, G. Liu, and Z. Peng, Trans. Nonferrous Met. Soc. 30, 1980. (2020).
A. Bhattacharya, and P.M. Singh, Corros. Sci. 53, 71. (2011).
D. Tromans, J. Electrochem. Soc. 127, 1253. (1980).
P.E. Hazlewood, P.M. Singh, and J.S. Hsieh, Ind. Eng. Chem. Res. 45, 7789. (2006).
R.J. Biernat, and R.G. Robins, Electrochim. Acta 17, 1261. (1972).
Acknowledgements
The authors gratefully acknowledge the National Natural Science Foundation of China (Grant Nos. 51804142 and 51604309) and the Doctoral Scientific Research Foundation of Jiangxi University of Science and Technology (jxxjbs17077) for financial support.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wang, S., Niu, F., Wang, Y. et al. Effects of \({{{S}}^{{{2} - }}}\)- and \({{S_2}O_3^{2 - }}\)-Containing Bayer Solutions on Corrosion of 16Mn Low-Alloy Steel at Elevated Temperatures. JOM 73, 3920–3927 (2021). https://doi.org/10.1007/s11837-021-04918-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11837-021-04918-1