Abstract
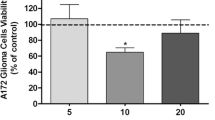
Glioblastoma (GBM) is the most malignant and deadly brain tumor. GBM cells overexpress the CD73 enzyme, which controls the level of extracellular adenosine, an immunosuppressive molecule. Studies have shown that some nonsteroidal anti-inflammatory drugs (NSAIDs) and methotrexate (MTX) have antiproliferative and modulatory effects on CD73 in vitro and in vivo. However, it remains unclear whether the antiproliferative effects of MTX and NSAIDS in GBM cells are mediated by increases in CD73 expression and adenosine formation. The aim of this study was to evaluate the effect of the NSAIDs, naproxen, piroxicam, meloxicam, ibuprofen, sodium diclofenac, acetylsalicylic acid, nimesulide, and ketoprofen on CD73 expression in GBM and mononuclear cells. In addition, we sought to understand whether the effects of MTX may be mediated by CD73 expression and activity. Cell viability and CD73 expression were evaluated in C6 and mononuclear cells after exposure to NSAIDs. For analysis of the mechanism of action of MTX, GBM cells were treated with APCP (CD73 inhibitor), dipyridamole (inhibitor of adenosine uptake), ABT-702 (adenosine kinase enzyme inhibitor), or caffeine (P1 adenosine receptor antagonist), before treatment with MTX and AMP, in the presence or not of mononuclear cells. In summary, only MTX increased the expression of CD73 in GBM cells decreasing cells viability by mechanisms independent of the adenosinergic system. Further studies are needed to understand the role of MTX in the GBM microenvironment.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Siegel RL, Miller KD, Jemal A (2020) Cancer statistics, 2020. CA Cancer J Clin 70(1):7–30. https://doi.org/10.3322/caac.21590
Perry A, Wesseling P (2016) Histologic classification of gliomas. Handb Clin Neurol 134:71–95. https://doi.org/10.1016/B978-0-12-802997-8.00005-0
Hanahan D, Coussens LM (2012) Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 21:309–322. https://doi.org/10.1016/j.ccr.2012.02.022
Aldape K, Zadeh G, Mansouri S, Reifenberger G, von Deimling A (2015) Glioblastoma: pathology, molecular mechanisms and markers. Acta Neuropathol 129(6):829–848. https://doi.org/. https://doi.org/10.1007/s00401-015-1432-1
Schneider T, Mawrin C, Scherlach C, Skalej M, Firsching R (2010) Gliomas in adults. Dtsch Arztebl Int 107(45):799–807; quiz 808. https://doi.org/10.3238/arztebl.2010.0799
Mello PA, Coutinho-Silva R, Savio LEB (2017) Multifaceted effects of extracellular adenosine triphosphate and adenosine in the tumor-host interaction and therapeutic perspectives. Front Immunol 8:1–17. https://doi.org/10.3389/fimmu.2017.01526
Bastid J, Cottalorda-Regairaz A, Alberici G, Bonnefoy N, Eliaou JF, Bensussan A (2012) ENTPD1/CD39 is a promising therapeutic target in oncology. Oncogene 32(14):1743–1751. https://doi.org/10.1038/onc.2012.269
Borsellino G, Kleinewietfeld M, Di Mitri D et al (2007) Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: hydrolysis of extracellular ATP and immune suppression. Blood 110(4):1225–1232. https://doi.org/10.1182/blood-2006-12-064527
Albesiano E, Han JE, Lim M (2010) Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am 21(1):17–29. https://doi.org/10.1016/j.nec.2009.08.008
Bavaresco L, Bernardi A, Braganhol E, Cappellari AR, Rockenbach L, Farias PF, Wink MR, Delgado-Cañedo A, Battastini AMO (2008) The role of ecto-5′-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem 319(1-2):61–68. https://doi.org/10.1007/s11010-008-9877-3
Azambuja JH, Gelsleichter NE, Beckenkamp LR, Iser IC, Fernandes MC, Figueiró F, Battastini AMO, Scholl JN, de Oliveira FH, Spanevello RM, Sévigny J, Wink MR, Stefani MA, Teixeira HF, Braganhol E (2019) CD73 downregulation decreases in vitro and in vivo glioblastoma growth. Mol Neurobiol 56:3260–3279. https://doi.org/10.1007/s12035-018-1240-4
Xu S, Shao QQ, Sun JT, Yang N, Xie Q, Wang DH, Huang QB, Huang B, Wang XY, Li XG, Qu X (2013) Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro Oncol 15(9):1160–1172. https://doi.org/10.1093/neuonc/not067
Rainsford KD (2007) Anti-inflammatory drugs in the 21st century. Subcell Biochem 42:3–27. https://doi.org/10.1007/1-4020-5688-5_1
Carvalho AW, Carvalho RDS, Rios-Santos F (2004) Specific cyclooxygenase-2 inhibitor analgesics: therapeutic advances. Rev Bras Anestesiol 54(3):448–464. https://doi.org/10.1590/S0034-70942004000300017
Williamson T, Bai RY, Staedtke V et al (2016) Mebendazole and a non-steroidal anti-inflammatory combine to reduce tumor initiation in a colon cancer preclinical model. Oncotarget 7(42):68571–68584. https://doi.org/10.18632/oncotarget.11851
Baek SJ (2002) Dual function of nonsteroidal anti-inflammatory drugs (NSAIDs): inhibition of cyclooxygenase and induction of NSAID-activated gene. J Pharmacol Exp Ther 301(3):1126–1131. https://doi.org/10.1124/jpet.301.3.1126
Bernardi A, Bavaresco L, Wink MR, Jacques-Silva MC, Delgado-Cañedo A, Lenz G, Battastini AMO (2007) Indomethacin stimulates activity and expression of ecto5′-nucleotidase/CD73 in glioma cell lines. Eur J Pharmacol 569(1-2):8–15. https://doi.org/10.1016/j.ejphar.2007.04.058
Luedi MM, Singh SK, Mosley JC, Hassan ISA, Hatami M, Gumin J, Andereggen L, Sulman EP, Lang FF, Stueber F, Fuller GN, Colen RR, Zinn PO (2018) Dexamethasone-mediated oncogenicity in vitro and in an animal model of glioblastoma. J Neurosurg 129:1446–1455. https://doi.org/10.3171/2017.7.JNS17668
McHugh CI, Thipparthi MR, Lawhorn-Crews JM et al (2018) Using radiolabeled 3′-deoxy-3′-18F-fluorothymidine with PET to monitor the effect of dexamethasone on non-small cell lung cancer. J Nucl Med 59:1544–1550. https://doi.org/10.2967/jnumed.117.207258
Wang H, Li M, Rinehart JJ, Zhang R (2004) Pretreatment with dexamethasone increases antitumor activity of carboplatin and gemcitabine in mice bearing human cancer xenografts: in vivo activity, pharmacokinetics, and clinical implications for cancer chemotherapy. Clin Cancer Res 10(5):1633–1644. https://doi.org/10.1158/1078-0432.ccr-0829-3
Wang H, Wang Y, Rayburn ER, Hill D, Rinehart J, Zhang R (2007) Dexamethasone as a chemosensitizer for breast cancer chemotherapy: potentiation of the antitumor activity of adriamycin, modulation of cytokine expression, and pharmacokinetics. Int J Oncol 30(4):947–953. https://doi.org/10.3892/ijo.30.4.947
Bavaresco L, Bernardi A, Braganhol E, Wink MR, Battastini AMO (2007) Dexamethasone inhibits proliferation and stimulates ecto-5′- nucleotidase/CD73 activity in C6 rat glioma cell line. J Neurooncol 84(1):1–8. https://doi.org/10.1007/s11060-007-9342-2
Jeibouei S, Akbari ME, Kalbasi A, Aref A, Ajoudanian M, Rezvani A, Zali H (2019) Personalized medicine in breast cancer: pharmacogenomics approaches. Pharmgenomics Pers Med 12:59–73. https://doi.org/10.2147/PGPM.S167886
Patel JN (2016) Cancer pharmacogenomics, challenges in implementation, and patient-focused perspectives. Pharmgenomics Pers Med 9:65–77. https://doi.org/10.2147/PGPM.S62918
Purcell WT, Ettinger DS (2003) Novel antifolate drugs. Curr Oncol Rep 5(2):114–125. https://doi.org/10.1007/s11912-003-0098-3
Mcguire JJ (2003) Anticancer antifolates: current status and future directions. Curr Pharm Des 9(31):2593–2613. https://doi.org/10.2174/1381612033453712
Grim J, Chládek J, Martínková J (2003) Pharmacokinetics and pharmacodynamics of methotrexate in non-neoplastic diseases. Clin Pharmacokinet 42(2):139–151. https://doi.org/10.2165/00003088-200342020-00003
Huennekens FM (1994) The methotrexate story: a paradigm for development of cancer chemotherapeutic agents. Adv Enzyme Regul 34:397–419. https://doi.org/10.1016/0065-2571(94)90025-6
Figueiró F, de Oliveira CP, Bergamin LS, Rockenbach L, Mendes FB, Jandrey EHF, Moritz CEJ, Pettenuzzo LF, Sévigny J, Guterres SS, Pohlmann AR, Battastini AMO (2016) Methotrexate up-regulates ecto-5′-nucleotidase/CD73 and reduces the frequency of T lymphocytes in the glioblastoma microenvironment. Purinergic Signal 12(2):303–312. https://doi.org/10.1007/s11302-016-9505-8
Scherer EB, Savio LE, Vuaden FC, Ferreira AG, Bogo MR, Bonan CD, Wyse AT (2012) Chronic mild hyperhomocysteinemia alters ectonucleotidase activities and gene expression of ecto-5′-nucleotidase/CD73 in rat lymphocytes. Mol Cell Biochem 362(1-2):187–194. https://doi.org/10.1007/s11010-011-1141-6
Butowski NA, Sneed PK, Chang SM (2006) Diagnosis and treatment of recurrent high-grade astrocytoma. J Clin Oncol 24(8):1273–1280. https://doi.org/10.1200/JCO.2005.04.7522
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P (2007) (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114(2):97–109. https://doi.org/10.1007/s00401-007-0243-4
Lapointe S, Perry A, Butowski NA (2018) Primary brain tumours in adults. Lancet 392(10145):432–446. https://doi.org/10.1016/S0140-6736(18)30990-5
Watters JJ, Schartner JM, Badie B (2005) Microglia function in brain tumors. J Neurosci Res 81(3):447–455. https://doi.org/10.1002/jnr.20485
da Silveira EF, Chassot JM, Teixeira FC, Azambuja JH, Debom G, Beira FT, del Pino FAB, Lourenço A, Horn AP, Cruz L, Spanevello RM, Braganhol E (2013) Ketoprofen-loaded polymeric nanocapsules selectively inhibit cancer cell growth in vitro and in preclinical model of glioblastoma multiforme. Invest New Drugs 31(6):1424–1435. https://doi.org/10.1007/s10637-013-0016-y
Zimmermann H (2000) Extracellular metabolism of ATP and other nucleotides. Naunyn Schmiedebergs Arch Pharmacol 362(4-5):299–309. https://doi.org/. https://doi.org/10.1007/s002100000309
Wang R, Zhang Y, Lin X et al (2017) Prognositic value of CD73-adenosinergic pathway in solid tumor: a meta-analysis and systematic review. Oncotarget 8(34):57327–57336. https://doi.org/10.18632/oncotarget.16905
Figueiró F, de Oliveira CP, Rockenbach L, Mendes FB, Bergamin LS, Jandrey EHF, Edelweiss MI, Guterres SS, Pohlmann AR, Battastini AMO (2015) Pharmacological improvement and preclinical evaluation of methotrexate-loaded lipid-core nanocapsules in a glioblastoma model. J Biomed Nanotechnol 11(10):1808–1818. https://doi.org/10.1166/jbn.2015.2125
Shevchenko I, Mathes A, Groth C, Karakhanova S, Müller V, Utikal J, Werner J, Bazhin AV, Umansky V (2020) Enhanced expression of CD39 and CD73 on T cells in the regulation of anti-tumor immune responses. OncoImmunology 9(1):1744946. https://doi.org/10.1080/2162402X.2020.1744946T
Wang C, Lin W, Playa H, Sun S, Cameron K, Buolamwini JK (2013) Dipyridamole analogs as pharmacological inhibitors of equilibrative nucleoside transporters. Identification of novel potent and selective inhibitors of the 55 adenosine transporter function of human equilibrative nucleoside transporter 4 (hENT4). Biochem Pharmacol 86(11):1531–1540. https://doi.org/10.1016/j.bcp.2013.08.063
Boyle DL, Kowaluk EA, Jarvis MF, Lee CH, Bhagwat SS, Williams M, Firestein GS (2001) Anti-inflammatory effects of ABT-702, a novel nonnucleoside adenosine kinase inhibitor, in rat adjuvant arthritis. J Pharmacol Exp Ther 296(2):495–500
Solinas G, Germano G, Mantovani A, Allavena P (2009 Nov) (2009) Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol 86(5):1065–1073. https://doi.org/10.1189/jlb.0609385
Balkwill F, Coussens LM (2004) Cancer: an inflammatory link. Nature 431(7007):405–406. https://doi.org/10.1038/431405a
Nieto-Sampedro M, Valle-Argos B, Gómez-Nicola D, Fernández-Mayoralas A, Nieto-Díaz M (2011) Inhibitors of glioma growth that reveal the tumour to the immune system. Clin Med Insights Oncol 5:265–314. https://doi.org/10.4137/CMO.S7685
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359(5):492–507. https://doi.org/10.1056/NEJMra0708126
Funding
This study was supported by the following Brazilian agencies: F.F. and A.M.O.B. received support from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/PQ no. 302879/2017-0), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS/PQG numbers: 17/2551- 0000 970-3 and 19/2551-0001783-9 and FAPERGS/PRONEX - project number 16/2551- 0000473-0), and Instituto Nacional de Ciência e Tecnologia (INCT; INCT/CNPq/CAPES/FAPERGS no. 465671/2014-4). A.F.D., L.F.L.S., and J.N.S. were recipients of CNPq fellowship. J.S. received support from the Natural Sciences and Engineering Research Council of Canada (NSERC; RGPIN-2016-05867) and was the recipient of a “Chercheur National” Scholarship from the Fonds de Recherche du Québec – Santé (FRQS).
Author information
Authors and Affiliations
Contributions
The investigation was planned by D.V.L. and F.F. Cell culture experiments were performed by D.V.L., A.F.D., J.N.S., and L.F.L.S. with guidance from F.F and A.M.O.B. The anti-rat CD73 antibody was donated by J.S. Data processing, and the writing of this manuscript was performed by A.F.D. and L.F.L.S. with input from all co-authors. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Daniela Vasconcelos Lopes declares no competing interests.
Amanda de Fraga Dias declares no competing interests.
Luiz Fernando Lopes Silva declares no competing interests.
Juliete Nathali Scholl declares no competing interests.
Jean Sévigny declares no competing interests.
Ana Maria Oliveira Battastini declares no competing interests.
Fabrício Figueiró declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopes, D.V., de Fraga Dias, A., Silva, L.F.L. et al. Influence of NSAIDs and methotrexate on CD73 expression and glioma cell growth. Purinergic Signalling 17, 273–284 (2021). https://doi.org/10.1007/s11302-021-09775-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-021-09775-w