Abstract
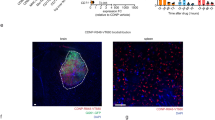
Glioblastoma multiforme (GBM) is a deadly cancer characterized by a pro-tumoral immune response. T-regulatory (Treg) lymphocytes suppress effector immune cells through cytokine secretion and the adenosinergic system. Ecto-5′-nucleotidase/CD73 plays a crucial role in Treg-mediated immunosuppression in the GBM microenvironment (GME). Methotrexate (MTX) is an immunosuppressive drug that can increase the extracellular concentration of adenosine. In this manuscript, C6 GBM cells were treated with 1.0 μM MTX, and ecto-5′-nucleotidase/CD73 expression and extracellular AMP metabolism were analyzed in vitro. For in vivo studies, rats with implanted GBM were treated for 10 days with MTX-loaded lipid-core nanocapsules (MTX-LNCs, 1 mg/kg/day). The activity of ectonucleotidase and the expression of NTPDase1/CD39 and ecto-5′-nucleotidase/CD73 were measured. The frequencies of T lymphocytes (CD3+CD4+, CD3+CD8+, and CD4+CD25highCD39+) were quantified. In vitro, treatment with MTX increased CD73 expression and activity in C6 cells, which is in agreement with higher levels of extracellular adenosine. In vivo, MTX-LNC treatment increased CD39 expression on CD3+CD8+ lymphocytes. In addition, MTX-LNC treatment up-regulated CD73 expression in tissue isolated from GBM, a finding that is in agreement with the higher activity of this enzyme. More specifically, the treatment increased CD73 expression on CD3+CD4+ and CD3+CD8+ lymphocytes. Treatment with MTX-LNCs decreased the frequencies of T-cytotoxic, T-helper, and Treg lymphocytes in the GME. Although more studies are necessary to better understand the complex cross-talk mediated by supra-physiological concentrations of adenosine in the GME, these studies demonstrate that MTX treatment increases CD73 enzyme expression and AMP hydrolysis, leading to an increase in adenosine production and immunosuppressive capability.





Similar content being viewed by others
References
Nieto-Sampedro M, Valle-Argos B, Gomez-Nicola D, Fernandez-Mayoralas A, Nieto-Diaz M (2011) Inhibitors of glioma growth that reveal the tumour to the immune system. Clin Med Insights Oncol 5:265–314. doi:10.4137/CMO.S7685cmo-5-2011-265
Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ (2010) Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin 60(3):166–193. doi:10.3322/caac.2006960/3/166
Pointer KB, Clark PA, Zorniak M, Alrfaei BM, Kuo JS (2014) Glioblastoma cancer stem cells: biomarker and therapeutic advances. Neurochem Int 71:1–7. doi:10.1016/j.neuint.2014.03.005S0197-0186(14)00052-7
Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, Sampson JH, Mitchell DA (2015) Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother 64(4):419–427. doi:10.1007/s00262-014-1651-7
Xu S, Shao QQ, Sun JT, Yang N, Xie Q, Wang DH, Huang QB, Huang B, Wang XY, Li XG, Qu X (2013) Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro Oncol 15(9):1160–1172. doi:10.1093/neuonc/not067not067
Kumar V (2013) Adenosine as an endogenous immunoregulator in cancer pathogenesis: where to go? Purinergic Signal 9(2):145–165. doi:10.1007/s11302-012-9349-9
Stagg J, Smyth MJ (2010) Extracellular adenosine triphosphate and adenosine in cancer. Oncogene 29(39):5346–5358. doi:10.1038/onc.2010.292onc2010292
Cekic C, Linden J (2014) Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res 74(24):7239–7249. doi:10.1158/0008-5472.CAN-13-35810008-5472.CAN-13-3581
Muller-Haegele S, Muller L, Whiteside TL (2014) Immunoregulatory activity of adenosine and its role in human cancer progression. Expert Rev Clin Immunol 10(7):897–914. doi:10.1586/1744666X.2014.915739
Sitkovsky MV (2009) T regulatory cells: hypoxia-adenosinergic suppression and re-direction of the immune response. Trends Immunol 30(3):102–108. doi:10.1016/j.it.2008.12.002S1471-4906(09)00002-7
Albesiano E, Han JE, Lim M (2010) Mechanisms of local immunoresistance in glioma. Neurosurg Clin N Am 21(1):17–29. doi:10.1016/j.nec.2009.08.008S1042-3680(09)00078-3
Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B (2007) Metronomic cyclophosphamide regimen selectively depletes CD4+ CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother 56(5):641–648. doi:10.1007/s00262-006-0225-8
Bernardi A, Bavaresco L, Wink MR, Jacques-Silva MC, Delgado-Canedo A, Lenz G, Battastini AM (2007) Indomethacin stimulates activity and expression of ecto-5′-nucleotidase/CD73 in glioma cell lines. Eur J Pharmacol 569(1–2):8–15. doi:10.1016/j.ejphar.2007.04.058
Supernat A, Markiewicz A, Welnicka-Jaskiewicz M, Seroczynska B, Skokowski J, Sejda A, Szade J, Czapiewski P, Biernat W, Zaczek A (2012) CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol 20(2):103–107
McGuire JJ (2003) Anticancer antifolates: current status and future directions. Curr Pharm Des 9(31):2593–2613
Abolmaali SS, Tamaddon AM, Dinarvand R (2013) A review of therapeutic challenges and achievements of methotrexate delivery systems for treatment of cancer and rheumatoid arthritis. Cancer Chemother Pharmacol 71(5):1115–1130. doi:10.1007/s00280-012-2062-0
Tohyama N, Tanaka S, Onda K, Sugiyama K, Hirano T (2013) Influence of anticancer agents on cell survival, proliferation, and CD4 + CD25 + Foxp3+ regulatory T cell-frequency in human peripheral-blood mononuclear cells activated by T cell-mitogen. Int Immunopharmacol 15(1):160–166. doi:10.1016/j.intimp.2012.11.008S1567-5769(12)00329-3
Morabito L, Montesinos MC, Schreibman DM, Balter L, Thompson LF, Resta R, Carlin G, Huie MA, Cronstein BN (1998) Methotrexate and sulfasalazine promote adenosine release by a mechanism that requires ecto-5′-nucleotidase-mediated conversion of adenine nucleotides. J Clin Invest 101(2):295–300. doi:10.1172/JCI1554
Montesinos MC, Takedachi M, Thompson LF, Wilder TF, Fernandez P, Cronstein BN (2007) The antiinflammatory mechanism of methotrexate depends on extracellular conversion of adenine nucleotides to adenosine by ecto-5′-nucleotidase: findings in a study of ecto-5′-nucleotidase gene-deficient mice. Arthritis Rheum 56(5):1440–1445. doi:10.1002/art.22643
Wolff JE, Kortmann RD, Wolff B, Pietsch T, Peters O, Schmid HJ, Rutkowski S, Warmuth-Metz M, Kramm C (2011) High dose methotrexate for pediatric high grade glioma: results of the HIT-GBM-D pilot study. J Neurooncol 102(3):433–442. doi:10.1007/s11060-010-0334-2
Figueiró F, Oliveira CP, Rockenbach L, Mendes FB, Bergamin L, Jandrey EHF, Edelweiss MI, Guterres SS, Pohlmann AR, Battastini AM (2015) Pharmacological improvement and preclinical evaluation of methotrexate-loaded lipid-core nanocapsules in a glioblastoma model. J Biomed Nanotechnol 11(10):1808–1818
Wink MR, Lenz G, Braganhol E, Tamajusuku AS, Schwartsmann G, Sarkis JJ, Battastini AM (2003) Altered extracellular ATP, ADP and AMP catabolism in glioma cell lines. Cancer Lett 198(2):211–218
Chan KM, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+-stimulated ATPase activity. Anal Biochem 157(2):375–380
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Voelter W, Zech K, Arnold P, Ludwig G (1980) Determination of selected pyrimidines, purines and their metabolites in serum and urine by reversed-phase ion-pair chromatography. J Chromatogr 199:345–354
Figueiro F, Bernardi A, Frozza RL, Terroso T, Zanotto-Filho A, Jandrey EH, Moreira JC, Salbego CG, Edelweiss MI, Pohlmann AR, Guterres SS, Battastini AM (2013) Resveratrol-loaded lipid-core nanocapsules treatment reduces in vitro and in vivo glioma growth. J Biomed Nanotechnol 9(3):516–526
Takano T, Lin JH, Arcuino G, Gao Q, Yang J, Nedergaard M (2001) Glutamate release promotes growth of malignant gliomas. Nat Med 7(9):1010–1015
Mandapathil M, Lang S, Gorelik E, Whiteside TL (2009) Isolation of functional human regulatory T cells (Treg) from the peripheral blood based on the CD39 expression. J Immunol Methods 346(1–2):55–63. doi:10.1016/j.jim.2009.05.004S0022-1759(09)00153-7
Prados MD, Byron SA, Tran NL, Phillips JJ, Molinaro AM, Ligon KL, Wen PY, Kuhn JG, Mellinghoff IK, de Groot JF, Colman H, Cloughesy TF, Chang SM, Ryken TC, Tembe WD, Kiefer JA, Berens ME, Craig DW, Carpten JD, Trent JM (2015) Toward precision medicine in glioblastoma: the promise and the challenges. Neuro Oncol. doi:10.1093/neuonc/nov031
Rolle CE, Sengupta S, Lesniak MS (2012) Mechanisms of immune evasion by gliomas. Adv Exp Med Biol 746:53–76. doi:10.1007/978-1-4614-3146-6_5
Kim TH, Kim YK, Woo JS (2012) The adenosine A3 receptor agonist Cl-IB-MECA induces cell death through Ca(2)(+)/ROS-dependent down regulation of ERK and Akt in A172 human glioma cells. Neurochem Res 37(12):2667–2677. doi:10.1007/s11064-012-0855-5
Corbelini PF, Figueiro F, das Neves GM, Andrade S, Kawano DF, Oliveira Battastini AM, Eifler-Lima VL (2015) Insights into ecto-5′-nucleotidase as a new target for cancer therapy: a medicinal chemistry study. Curr Med Chem
Gouttefangeas C, Mansur I, Schmid M, Dastot H, Gelin C, Mahouy G, Boumsell L, Bensussan A (1992) The CD39 molecule defines distinct cytotoxic subsets within alloactivated human CD8-positive cells. Eur J Immunol 22(10):2681–2685. doi:10.1002/eji.1830221031
Parodi A, Battaglia F, Kalli F, Ferrera F, Conteduca G, Tardito S, Stringara S, Ivaldi F, Negrini S, Borgonovo G, Simonato A, Traverso P, Carmignani G, Fenoglio D, Filaci G (2013) CD39 is highly involved in mediating the suppression activity of tumor-infiltrating CD8+ T regulatory lymphocytes. Cancer Immunol Immunother 62(5):851–862. doi:10.1007/s00262-013-1392-z
Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL (2009) Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res 15(20):6348–6357. doi:10.1158/1078-0432.CCR-09-11431078-0432.CCR-09-1143
Su Y, Huang X, Raskovalova T, Zacharia L, Lokshin A, Jackson E, Gorelik E (2008) Cooperation of adenosine and prostaglandin E2 (PGE2) in amplification of cAMP-PKA signaling and immunosuppression. Cancer Immunol Immunother 57(11):1611–1623. doi:10.1007/s00262-008-0494-5
Hausler SF, Montalban del Barrio I, Strohschein J, Anoop Chandran P, Engel JB, Honig A, Ossadnik M, Horn E, Fischer B, Krockenberger M, Heuer S, Seida AA, Junker M, Kneitz H, Kloor D, Klotz KN, Dietl J, Wischhusen J (2011) Ectonucleotidases CD39 and CD73 on OvCA cells are potent adenosine-generating enzymes responsible for adenosine receptor 2A-dependent suppression of T cell function and NK cell cytotoxicity. Cancer Immunol Immunother 60(10):1405–1418. doi:10.1007/s00262-011-1040-4
Colombo MP, Piconese S (2007) Regulatory T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer 7(11):880–887. doi:10.1038/nrc2250
Cabrera R, Ararat M, Xu Y, Brusko T, Wasserfall C, Atkinson MA, Chang LJ, Liu C, Nelson DR (2012) Immune modulation of effector CD4+ and regulatory T cell function by sorafenib in patients with hepatocellular carcinoma. Cancer Immunol Immunother 62(4):737–746. doi:10.1007/s00262-012-1380-8
Zhao G-J, Lu Z-Q, Tang L-M, Wu Z-S, Wang D-W, Zheng J-Y, Qiu Q-M (2012) Curcumin inhibits suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. Int Immunopharmacol 14(1):99–106. doi:10.1016/j.intimp.2012.06.016
Majumdar S, Aggarwal BB (2001) Methotrexate suppresses NF-kappaB activation through inhibition of IkappaBalpha phosphorylation and degradation. J Immunol 167(5):2911–2920
Mediero A, Perez-Aso M, Cronstein BN (2013) Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br J Pharmacol 169(6):1372–1388. doi:10.1111/bph.12227
Acknowledgments
This study was supported by the following Brazilian agencies: Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Proc. 472577/2013-1, Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (Capes), and Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All of the procedures used in the present study followed the “Principles of Laboratory Animal Care” from the National Institutes of Health (NIH) and were approved by the Ethical Committee of the Universidade Federal do Rio Grande do Sul (Protocol # 26389).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Figueiró, F., de Oliveira, C.P., Bergamin, L.S. et al. Methotrexate up-regulates ecto-5′-nucleotidase/CD73 and reduces the frequency of T lymphocytes in the glioblastoma microenvironment. Purinergic Signalling 12, 303–312 (2016). https://doi.org/10.1007/s11302-016-9505-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11302-016-9505-8