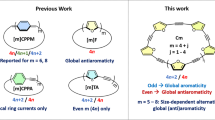
Abstract
The maleimide moiety is present in numerous species of commercial or theoretical interest. The resemblance of maleimide to aromatic pyrrole raise the question of the aromaticity of the former. Are maleimides aromatic or antiaromatic, or neither? Should they be described as N-substituted derivatives of (2,5-dihydro)pyrrole-2,5-dione or as pyrrole-2,5-dioxy biradical? Through an analysis of the energetics of neutral and ionic species alike, the former description is to be preferred with only minimal antiaromaticity associated with its 4 π electrons.





Similar content being viewed by others
Data availability
Not applicable.
Code applicability
Code applicability
References
Gaisina IN, Gallier F, Ougolkov AV, Kim KH, Kurome T, Guo S, Holzle D, Luchini DN, Blond SY, Billadeau DD, Kozikowski AP (2009) From a natural product lead to the identification of potent and selective benzofuran-3-yl-(indol-3-yl)maleimides as glycogen synthese kinase 3b inhibitors that suppress proliferation and survival of pancereatic cancer cells. J Med Chem 52:1853–1863. https://doi.org/10.1021/jm801317h
Wagner J, von Matt SR, Albert R, Cooke N, Ehrhardt C, Geiser M, Rummel G, Stark W, Strauss A, Cowan-Jacob SW, Beerli C, Weckbecker G, Evanou JP, Zenke G, Cottens S (2009) Discovery of 3-(1H-indol-3-yl)-4-[2-(4-methylpiperazin-1-yl)quinazolin-4-yl]-pyrrole-2,5-dione (AEB071), a potent and selective inhibitor of protein kinase C isotypes. J Med Chem 52:6193–6196. https://doi.org/10.1021/jm901108b
Gunosewoyo H, Midzak A, Gaisina IN, Sabath EV, Fedolak A, Hanania T, Brunner D, Papadopoulos V, Kozikowski AP (2013) Characterization of maleimide-based glycogen synthase kinase-3(GSK-3) inhibitors as stimulators of stereoidogenesis. J Med Chem 56:5115–5129. https://doi.org/10.1021/jm400511s
Zhang M, Li B, Chen H, Lu H, Ma H, Cheng X, Wang W, Wang Y, Ding Y, Hu A (2020) Triggering the antitumor activity of acyclic enediyne through maleimide-assisted rearrangement and cycloaromatization. J Org Chem 85:9808–9819. https://doi.org/10.1021/acs.joc.0c01124
Chen Y, Tsao K, Acton SL, Keillor JW (2018) A green BODIPY-based, super-fluorogenic, protein-specific labelling agent. Angew Chem Int Ed 57:12390–12394. https://doi.org/10.1002/anie.201805482
Matsumoto T, Urano Y, Shoda T, Kojima H, Nagano T (2007) A thiol-reactive fluorescence probe based on donor-excited photoinduced electron transfer: key role of ortho substitution. Org Lett 9:3375–3377. https://doi.org/10.1021/ol071352e
Kand D, Kalle AM, Varma SJ, Talukdar PA (2012) Chromenoquinoline-based fluorescent off-on thiol probe for bioimaging. Chem Commun 48:2722–2742. https://doi.org/10.1039/C2CC16593G
Chen Y, Parr T, Holmes AE, Nakanishi K (2008) Porphyrinmaleimides: towards thiol probes for cysteine residues in proteins. Bioconjugate Chem 19:5–9. https://doi.org/10.1021/bc700267f
Yeh HC, Wu WC, Chen CT (2003) The colorful fluorescence from readily-synthesized 3,4-diaryl-substituted maleimide fluorophores. Chem Comm 404-405. https://doi.org/10.1039/B211537A
Nakazono M, Nanobu S, Uesaki A, Kuwano R, Kashiwabara M, Zaitsu K (2007) Bisindolylmaleimides with large Stokes shift and long-lasting chemiluminescence properties. Org. Lett. 9:3583–3586. https://doi.org/10.1021/ol701431g
Price J, Albright E, Decken A, Eisler S (2019) Thioarylamides: accessible, tunable, and strongly emissive building blocks. Org Biomol Chem 17:9562–9566. https://doi.org/10.1039/C9OB01741K
Yeh HC, Wu WC, Wen YS, Dai DC, Wang JK, Chen CT (2004) Derivatives od α,β-dicyanostilbene: Convenient precursor for the synthesis of diphenylmaleimide compounds, E-Z isomerization, crystal structure, and solid-state fluorescence. J Org Chem 69:6455–6462. https://doi.org/10.1021/jo049512c
Yang Z, Li X, Yang K, Yu N, Gao R, Ren Y (2023) Synthesis and unexpected optical properties of ionic phosphorus heterocycles with P-regulated noncovalent interactions. J Org Chem 88:2792–2800. https://doi.org/10.1021/acs.joc.2c02424
Xie HD, Ho LA, Truelove MS, Corry B, Stewart SG (2010) Fluorescent triphenyl substituted maleimide derivatives: synthesis, spectroscopy, and quantum chemical calculations. J Fluores 20:1077–1085. https://doi.org/10.1007/s10895-010-0660-y
Fukaminato T, Irie M (2006) Reversible fluorescence wavelength shift based on photoinduced aggregate formation. Adv Mater 18:3225–3228. https://doi.org/10.1002/adma.200601222
van Herpt JT, Stuart MCA, Browne WR, Feringa BL (2014) A dithienylethene-based rewritable hydrogelator. Chem Eur J 20:3077–3083. https://doi.org/10.1002/chem.201304064
Ohsumi M, Fukaminato T, Irie M (2005) Chemical control of the photochromic reactivity of diarylethene derivatives. Chem Comm 3921-3923. https://doi.org/10.1039/B506801K
Ravasco JM, Faustino H, Trindade A, Gois PMP (2019) Bioconjugation with maleimides: a useful tool for chemical biology. Chem Eur J 25:43–59. https://doi.org/10.1002/chem.201803174
Waghray D, Nulens W, Dehaen W (2011) Efficient synthesis of benzo fused tetrathia[7]helicenes. Org Lett 13:5516–5519. https://doi.org/10.1021/ol202236r
Bock H, Subervie D, Mathey P, Pradhan A, Sarkar P, Dechambenoit P, Hilard EA, Durola F (2014) Helicenes from diarylmaleimides. Org Lett 16:1546–1549. https://doi.org/10.1021/ol500154k
Ansteatt S, Gelfand R, Pelton M, Ptaszek M (2023) Geometry-independent ultrafast energy transfer in bioinspired arrays containing electronically coupled BODIPY dyads as energy donors. Chem Eur J, in press. https://doi.org/10.1002/chem.202301571
Ansteatt S, Uthe B, Mandal B, Gelfand R, Dunietz BD, Pelton M, Ptaszek M (2023) Engineering giant excitonic coupling in bioinspired, covalently bridged BODIPY dyads. Phys Chem Chem Phys 25:8013–8027. https://doi.org/10.1039/D2CP05621F
Rulisek L, Sebek P, Havlas Z, Hrabal R, Capek P, Svatos A (2005) An experimental and theoretical study of stereoselectivity of furan-maleic anhydride and furan-maleimide Diels-Alder reactions. J Org Chem 70:6295–6302. https://doi.org/10.1021/jo050759z
Yoshioka S, Aoyama H, Fujioka H, Arisawa M (2018) Cascade, multiple Diels-Alder reactions of styrene derivatives with maleimide or maleic anhydride. J Org Chem 83:6599–6606. https://doi.org/10.1021/acs.joc.8b00890
Gidron O, Shimon LJW, Leitus G, Bendikov M (2012) Reactivity of long conjugated systems: selectivity of Diels-Alder cycloaddition in oligofurans. Org Lett 14:502–505. https://doi.org/10.1021/ol202987e
Uchoa AF, de Oliveira KT, Baptista MS, Bortoluzzi AJ, Iamamoto Y, Serra OA (2011) Chlorin photosensitizers sterically designed to prevent self-aggregation. J Org Chem 76:8824–8832. https://doi.org/10.1021/jo201568n
Fallon T, Willis AC, Paddon-Row MN, Sherburn MS (2014) Furanodendralenes. J Org Chem 79:3185–3193. https://doi.org/10.1021/jo500458y
Werner S, Curran DP (2003) Fluorous dienophiles are powerful diene scavengers in Diels-Alder reactions. Org Lett 5:3293–3296. https://doi.org/10.1021/ol035214a
Zeng Z, Ishida M, Zafra JL, Zhu X, Sung YM, Bao N, Webster RD, Lee BS, Li RW, Zeng W, Li Y, Chi C, Navarrete JTL, Ding J, Casado J, Kim D, Wu J (2013) Pushing extended p-quinodimethanes to the limit: stable tetracyano-oligo(N-annulated perylene)quinodimethanes with tunable ground states. J Am Chem Soc 135:6363–6371. https://doi.org/10.1021/ja402467y
Zeng Z, Lee S, Zafra JL, Ishida M, Zhu X, Sun Z, Ni Y, Webster RD, Li RW, Navarrete JTL, Chi C, Dinh J, Casado J, Kim D, Wu J (2013) Tetracyanoqueterrylene and tetracyanohexarylenequinodimethanes with tunable ground states and strong near-infrared absorption. Angew Chem Int Ed 52:8561–8565. https://doi.org/10.1002/anie.201305348
Ishii A, Horikawa Y, Takaki I, Shibata J, Nakayama J, Hosh M (1991) Preparation of 2,5-bis(diarylmethylene)-2,5-dihydrothiophenes and their furan, selenophene, and N-methylpyrrole analogs. Tetrahedron Lett 32:4313–4316. https://doi.org/10.1016/S0040-4039(00)92158-0
Gouterman M (1978) Optical spectra and electronic structure of porphyrins and related rings. in The Porphyrins. Dolphin, D. Volume III, pages 1-156. Academic Press
Lindsey JS (2015) De novo synthesis of gem-dialkyl chlorophyll analogues for probing and emulating our green world. Chem Rev 115:6534–6620. https://doi.org/10.1021/acs.chemrev.5b00065
Brown KL (2005) Chemistry and enzymology of vitamin B12. Chem Rev 105:2075–2149. https://doi.org/10.1021/cr030720z
Eschenmoser A (1979) in Vitamin B12, ed. B. Zagalak and W. Friedrich, Walter de Gruyter, Berlin, 89–117
Mack J (2017) Expanded, contracted, and isomeric porphyrins: theoretical aspects. Chem Rev 117:3444–3478. https://doi.org/10.1021/acs.chemrev.6b00568
Bongards C, Gartner W (2010) The role of the chromophore in the biological photoreceptor phytochrome: an approach using chemically synthesized tetrapyrroles. Acc Chem Res 43:485–495. https://doi.org/10.1021/ar800133x
Tomat E, Curtis CJ (2021) Biopyrrin pigments: from heme metabolites to redox-active ligands and luminescent radicals. Acc Chem Res 54:4584–4594. https://doi.org/10.1021/acs.accounts.1c00613
Hammad LA, Wenthold PG (2000) Synthesis, characterization, and reactivity of the m-xylylene anion in the gas phase. The Enthalpy of formation of m-xylylene. J Am Chem Soc 122:11203–11211. https://doi.org/10.1021/ja960663+
Pei Z, Magann NL, Sowden MJ, Murphy RB, Gardiner MG, Sherburn MS, Coote ML (2023) Computational and experimental confirmation of the diradical character of para-quinonemethide. J Am Chem Soc ASAP Article. https://doi.org/10.1021/jacs.3c04363
Roux MV, Jiménez P, Martin-Luengo MA, Dávalos JZ, Sun Z, Hosmane RS, Liebman JF (1997) The elusive antiaromaticity of maleimides and maleic anhydride: Enthalpies of formation of N-methylmaleimide, N-methylsuccinimide, N-methylphthalimide and N-benzoyl-N-methylbenzamide. J Org Chem 62:2732–2737. https://doi.org/10.1021/jo9621985
Matos MAR, Liebman JF (2009) In Gupta RR, Krygowski TM, Cyranski MK (eds) Topics in heterocyclic chemistry: experimental thermochemistry of heterocycles and their aromaticity. A study of nitrogen, oxygen, and sulfur derivatives of indane and indene, vol 19. Springer, Heidelberg, 1–26. https://doi.org/10.1007/7081_2008_5
Miranda MS, Matos MAR, Morais VMF, Liebman JF (2011) Paradigms and paradoxes: The aromaticity of 6:6 fused carbocycles and heterocycles as an extension of a study of indane and indene derivatives. StructChem 22:1221-1224 (2011). https://doi.org/10.1007/s11224-011-9812-1
Fattahi A, Kass SR, Liebman JF, Matos MAR, Miranda MS, Morais VMF (2005) The enthalpies of formation of o-, m-and p-benzoquinone: gas-phase ion energetics, combustion calorimetry and quantum chemical computations combined. J Am Chem Soc 127:6116–6122. https://doi.org/10.1021/ja042612f
Emel'yanenko VN; Varfolomeev MA, Novikov VB, Turovtsev VV, Orlov YD (2017) Thermodynamic properties of 1,4-benzoquinones in gaseous and condensed phases: experimental and theoretical studies. J Chem Eng Data 62:2413-2422. https://doi.org/10.1021/acs.jced.7b00354
Gonçalves EM, Agapito F, Almeida TS, Martinho Simões JA (2014) Enthalpies of formation of dihydroxybenzenes revisited: combining experimental and high-level ab initio data. J Chem Thermodyn 73:90–99. https://doi.org/10.1016/j.jct.2013.10.032
Fu QA, Yang JL (2011) Wang XB (2001) On the electronic structures and electron affinities of the m-benzoquinone (BQ) diradical and the o-, p-BQ molecules: a synergetic photoelectron spectroscopic and theoretical study. J Phys Chem A 115:3201–3207. https://doi.org/10.1021/jp1120542
Paul G, Kebarle P (1989) Electron affinities of cyclic unsaturated dicarbonyls: maleic anhydrides, maleimides, and cyclopentenedione. J Am Chem Soc 111:464–470. https://doi.org/10.1021/ja00184a009
Shapiro R, Nesnow S (1969)1-Methylazepine-2,7-dione: synthesis and reactions, J Org Chem 34:1695-1700. https://doi.org/10.1021/jo01258a036
WvE Doering, Detert F (1951) Cycloheptatrienylium oxide. J Am Chem Soc 73:876–877
Turner RB, Meador WR, Doering WvE, Knox LH, Mayer JR, Wiley DW (1957) Heats of hydrogenation. III. Hydrogenation of cycloöctatetraene and of some seven-membered nonbenzenoid aromatic compounds, J Am Chem Soc 79:4127-4133. https://doi.org/10.1021/ja01572a041
Hess BA, Jr; Schaad LJ. Holyoke CW Jr, (1972) Aromaticity of annulenones. Tetrahedron 28:5299–5305
Fattahi A, Liebman JF, Miranda MS, Morais VMF, Matos MAR, Lis L, Kass SR (2014) Indenone and cyclopentadienone energetics via mass spectrometry and computations: are these species antiaromatic or ‘merely’ nonaromatic? Intl J Mass Spectry 369:87-91. https://doi.org/10.1016/j.ijms.2014.06.011. This paper was reprinted without change as Intl J Mass Spectry, 378:175-179 (2015). https://doi.org/10.1016/j.ijms.2014.07.042 as part of a special issue of the International Journal of Mass Spectrometry in honor of Prof. Veronica M. Bierbaum
Breugst M, Tokuyasu T, Mayr, H (2010) Nucleophilic reactivities of imide and amide anions. J Org Chem 75: 5250-5258. https://doi.org/10.1021/jo1009883
Acknowledgements
We also wish to thank Prof. Paul Smith for helpful comments on our study. Both authors are likewise grateful to the UMBC library science reference staff for supporting our extensive use of SciFindern.
Funding
M. P. gratefully acknowledges National Science Foundation (grant CHE-1955318).
Author information
Authors and Affiliations
Contributions
J. F. L. designed the study and wrote the manuscript, M. P. wrote the introduction and contributed to the study design.
Corresponding author
Ethics declarations
Ethical approval
We did not perform any experiments when preparing this article, so neither ethics review nor informed consent was necessary.
Consent to participate
Both authors agreed with participation in research and publication of the results.
Consent for publication
Both authors have approved the manuscript before submission, including the names and order of authors.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ptaszek, M., Liebman, J.F. Paradoxes and paradigms: are maleimides antiaromatic, aromatic, or neither?. Struct Chem 34, 2015–2019 (2023). https://doi.org/10.1007/s11224-023-02233-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02233-w