Abstract
Purpose
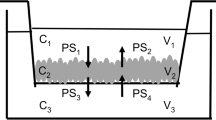
The purpose of this research is to analyze non-linear pharmacokinetics of P-glycoprotein (P-gp) substrates in a cell based assay of a microfluidic device, which might be affected by hydrodynamic barrier (unstirred water layer, UWL).
Results
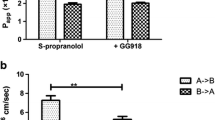
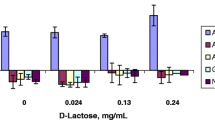
Apparent permeability (Papp) were obtained using non-P-gp substrates (propranolol, metoprolol, and atenolol) and P-gp substrates (quinidine and talinolol) in a commercially available microfluidic device, organoplate ® of Caco-2 cell based assay. The previous UWL resistance model was well fitted to Papp of static and flow condition by assuming UWL including and negligible condition, while P-gp substrates of higher passive permeability (quinidine) was apart from the fitting curve. The concentration dependent non-linear kinetics of P-gp substrates, quinidine and talinolol, was more analyzed in detail, and apparent Vmax discrepancy between static and flow assay condition in the quinidine assay was observed, while that was not observed in talinolol, the lower permeable substrate. Based on the experimental results, a mathematical model for P-gp substrates including UWL compartment on the previous 3-compartment model was developed, and it indicated that the apparent Vmax was variable along with the ratio between passive permeability and UWL permeability.
Conclusions
The mathematical model adding UWL compartment well explained non-linear pharmacokinetics of apparent permeability of P-gp substrate in the microfluidic device. The model also has a potential to be applied to P-gp substrate permeability analysis in vivo.






Similar content being viewed by others
Abbreviations
- Eq:
-
Equation
- Papp :
-
Apparent permeability
- P-gp:
-
P-glycoprotein
- UWL:
-
Unstirred water layer
- ZSQ:
-
Zosuquidar
References
Wetterich U, Spahn-Langguth H, Mutschler E, Terhaag B, Rosch W, Langguth P. Evidence for intestinal secretion as an additional clearance pathway of talinolol enantiomers: concentration- and dose-dependent absorption in vitro and in vivo. Pharm Res. 1996;13(4):514–22.
Caruso FS, Doshan HD, Hernandez PH, Costello R, Applin W, Neiss ES. Celiprolol: pharmacokinetics and duration of pharmacodynamic activity. British J Clin Practice Suppl. 1985;40:12–6.
Shirasaka Y, Masaoka Y, Kataoka M, Sakuma S, Yamashita S. Scaling of in vitro membrane permeability to predict P-glycoprotein-mediated drug absorption in vivo. Drug Metab Dispos Biol Fate Chem. 2008;36(5):916–22.
Tachibana T, Kitamura S, Kato M, Mitsui T, Shirasaka Y, Yamashita S, et al. Model analysis of the concentration-dependent permeability of P-gp substrates. Pharm Res. 2010;27(3):442–6.
Takano J, Maeda K, Bolger MB, Sugiyama Y. The prediction of the relative importance of CYP3A/P-glycoprotein to the nonlinear intestinal absorption of drugs by advanced compartmental absorption and transit model. Drug Metab Dispos Biol Fate Chem. 2016;44(11):1808–18.
Korjamo T, Heikkinen AT, Monkkonen J. Analysis of unstirred water layer in in vitro permeability experiments. J Pharm Sci. 2009;98(12):4469–79.
Lennernas H, Lee ID, Fagerholm U, Amidon GL. A residence-time distribution analysis of the hydrodynamics within the intestine in man during a regional single-pass perfusion with Loc-I-gut: in-vivo permeability estimation. J Pharm Pharmacol. 1997;49(7):682–6.
Johnson DA, Amidon GL. Determination of intrinsic membrane transport parameters from perfused intestine experiments: a boundary layer approach to estimating the aqueous and unbiased membrane permeabilities. J Theor Biol. 1988;131(1):93–106.
Lennernas H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica; Fate Foreign Compounds Biol Syst. 2007;37(10–11):1015–51.
Heikkinen AT, Korjamo T, Lepikkö V, Mönkkönen J. Effects of experimental setup on the apparent concentration dependency of active efflux transport in in vitroz cell permeation experiments. Mol Pharm. 2010;7(2):605–17.
Trietsch SJ, Naumovska E, Kurek D, Setyawati MC, Vormann MK, Wilschut KJ, et al. Membrane-free culture and real-time barrier integrity assessment of perfused intestinal epithelium tubes. Nat Commun. 2017;8(1):262.
Takano R, Furumoto K, Shiraki K, Takata N, Hayashi Y, Aso Y, et al. Rate-limiting steps of oral absorption for poorly water-soluble drugs in dogs; prediction from a miniscale dissolution test and a physiologically-based computer simulation. Pharm Res. 2008;25(10):2334–44.
Tavelin S, Gråsjö J, Taipalensuu J, Ocklind G, Artursson P. Applications of epithelial cell culture in studies of drug transport. Methods Mol Biol (Clifton, NJ). 2002;188:233–72.
Balakrishnan A, Hussainzada N, Gonzalez P, Bermejo M, Swaan PW, Polli JE. Bias in estimation of transporter kinetic parameters from overexpression systems: interplay of transporter expression level and substrate affinity. J Pharmacol Exp Ther. 2007;320(1):133–44.
Murgia X, Loretz B, Hartwig O, Hittinger M, Lehr CM. The role of mucus on drug transport and its potential to affect therapeutic outcomes. Adv Drug Deliv Rev. 2018;124:82–97.
Falavigna M, Stein PC, Flaten GE, di Cagno MP. Impact of Mucin on Drug Diffusion: Development of a Straightforward in Vitro Method for the Determination of Drug Diffusivity in the Presence of Mucin. Pharmaceutics. 2020;12(2).
Sigurdsson HH, Kirch J, Lehr CM. Mucus as a barrier to lipophilic drugs. Int J Pharm. 2013;453(1):56–64.
Acknowledgments and Dis1closures
The authors would like to thank Yuji Sakurai, Kazuhisa Ozeki, Jumpei Kiyokawa, Chisato Kaneko, Kai Tanaka, Mitsuyasu Tabo and Kimio Terao for their support of this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Igarashi, F., Nakagawa, T., Shinohara, Y. et al. Analysis of Non-linear Pharmacokinetics of P-Glycoprotein Substrates in a Microfluidic Device Using a Mathematical Model that Includes an Unstirred Water Layer (UWL) Compartment. Pharm Res 38, 1031–1039 (2021). https://doi.org/10.1007/s11095-021-03054-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-021-03054-4