Abstract
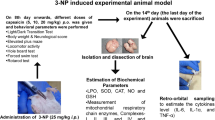
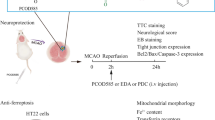
Various studies have evidenced the neuroprotective role of PDE4 inhibitors. However, whether PDE4 inhibitor, Piclamilast pharmacological post-treatment is protective during cerebral ischemia reperfusion-induced injury remains unknown. Therefore, this study design included testing the hypothesis that Piclamilast administered at the beginning of a reperfusion phase (Piclamilast pPost-trt) shows protective effects and explores & probes underlying downstream mechanisms. Swiss albino male mice were subjected to global ischemic and reperfusion injury for 17 min. The animals examined cerebral infarct size, biochemical parameters, inflammatory mediators, and motor coordination. For memory, assessment mice were subjected to morris water maze (MWM) and elevated plus maze (EPM) test. Histological changes were assessed using HE staining. Piclamilast pPost-trt significantly reduced I/R injury-induced deleterious effects on biochemical parameters of oxidative stress, inflammatory parameters, infarct size, and histopathological changes, according to the findings. These neuroprotective effects of pPost-trt are significantly abolished by pre-treatment with selective CREB inhibitor, 666–15. Current study concluded that induced neuroprotective benefits of Piclamilast Post-trt, in all probability, maybe mediated through CREB activation. Hence, its neuroprotective effects can be further explored in clinical settings.









Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article. There are no separate or additional files.
References
Global Health Estimates. Geneva: World Health Organization (2012). Available from: http://www.who.int/healthinfo/global_burden_disease/en/.
Schaller B, Graf R (2004) Cerebral ischemia and reperfusion: the pathophysiologic concept as a basis for clinical therapy. J Cereb Blood Flow Metab 24(4):351–371
Buchholz B, Donato M, D’Annunzio V, Gelpi RJ (2014) Ischemic postconditioning: mechanisms, comorbidities, and clinical application. Mol Cell Biochem 392(1):1–2
Khan H, Kashyap A, Kaur A, Singh TG (2020) Pharmacological postconditioning: a molecular aspect in ischemic injury. J Pharm Pharmacol 72(11):1513–1527
Penna C, Mancardi D, Rastaldo R, Losano G, Pagliaro P (2007) Intermittent activation of bradykinin B2 receptors and mitochondrial KATP channels trigger cardiac postconditioning through redox signaling. Cardiovasc Res 75(1):168–177
Tissier R, Waintraub X, Couvreur N, Gervais M, Bruneval P, Mandet C, Zini R, Enriquez B, Berdeaux A, Ghaleh B (2007) Pharmacological postconditioning with the phytoestrogen genistein. J Mol Cell Cardiol 42(1):79–87
Khan H, Singh A, Thapa K, Garg N, Grewal AK, Singh TG (2021) Therapeutic modulation of the phosphatidylinositol 3-kinases (PI3K) pathway in cerebral ischemic injury. Brain Res 1761:147399
Hein M, Zoremba N, Bleilevens C, Bruells C, Rossaint R, Roehl AB (2013) Levosimendan limits reperfusion injury in a rat middle cerebral artery occlusion (MCAO) model. BMC Neurol 13(1):1–8
Penna C, Perrelli MG, Tullio F, Angotti C, Camporeale A, Poli V, Pagliaro P (2013) Diazoxide postconditioning induces mitochondrial protein S-Nitrosylation and a redox-sensitive mitochondrial phosphorylation/translocation of RISK elements: no role for SAFE. Basic Res Cardiol 108(5):371
Bouhidel JO, Wang P, Li Q, Cai H (2014) Pharmacological postconditioning treatment of myocardial infarction with netrin-1. Front Biosci (Landmark edition) 19:566
Toyoda T, Tosaka S, Tosaka R, Maekawa T, Cho S, Eguchi S, Nakashima M, Sumikawa K (2014) Milrinone-induced postconditioning reduces hepatic ischemia-reperfusion injury in rats: the roles of phosphatidylinositol 3-kinase and nitric oxide. J Surg Res 186(1):446–451
Wu Y, Wan J, Zhen WZ, Chen LF, Zhan J, Ke JJ, Zhang ZZ, Wang YL (2014) The effect of butorphanol postconditioning on myocardial ischaemia reperfusion injury in rats. Interact Cardiovasc Thorac Surg 18(3):308–312
Grewal AK, Singh N, Singh TG (2019) Neuroprotective effect of pharmacological postconditioning on cerebral ischaemia–reperfusion-induced injury in mice. J Pharm Pharmacol 71(6):956–970
Grewal AK, Singh N, Singh TG (2019) Effects of resveratrol postconditioning on cerebral ischemia in mice: role of the sirtuin-1 pathway. Can J Physiol Pharmacol 97(11):1094–1101
Daicheng H, Shiwen X, Huaping Z, Yong L, Qianqian Z, Changxia H (2018) Fangchinoline ameliorates the expressions of angiogenic molecule in cerebral ischemia induced neuronal degeneration in neonatal rats. Transl Neurosci 9(1):117–122
Chen YC, Hsu WL, Ma YL, Tai DJ, Lee EH (2014) CREB SUMOylation by the E3 ligase PIAS1 enhances spatial memory. J Neurosci 34(29):9574–9589
Ortega-Martínez S (2015) A new perspective on the role of the CREB family of transcription factors in memory consolidation via adult hippocampal neurogenesis. Front Mol Neurosci 8:46
Pugazhenthi S, Wang M, Pham S, Sze CI, Eckman CB (2011) Downregulation of CREB expression in Alzheimer’s brain and in Aβ-treated rat hippocampal neurons. Mol Neurodegener 6(1):1–16
Andersson M, Konradi C, Cenci MA (2001) cAMP response element-binding protein is required for dopamine-dependent gene expression in the intact but not the dopamine-denervated striatum. J Neurosci 21(24):9930–9943
Choi YS, Lee B, Cho HY, Reyes IB, Pu XA, Saido TC, Hoyt KR, Obrietan K (2009) CREB is a key regulator of striatal vulnerability in chemical and genetic models of Huntington’s disease. Neurobiol Dis 36(2):259–268
Oger S, Méhats C, Dallot E, Cabrol D, Leroy MJ (2005) Evidence for a role of phosphodiesterase 4 in lipopolysaccharide-stimulated prostaglandin E2 production and matrix metalloproteinase-9 activity in human amniochorionic membranes. J Immunol 174(12):8082–8089
Baumer W, Hoppmann J, Rundfeldt C, Kietzmann M (2007) Highly selective phosphodiesterase 4 inhibitors for the treatment of allergic skin diseases and psoriasis. Inflamm Allergy Drug Targets 6(1):17–26
Richter W, Menniti FS, Zhang HT, Conti M (2013) PDE4 as a target for cognition enhancement. Expert Opin Ther Targets 17(9):1011–1027
Huang H, Hong Q, Tan HL, Xiao CR, Gao Y (2016) Ferulic acid prevents LPS-induced up-regulation of PDE4B and stimulates the cAMP/CREB signaling pathway in PC12 cells. Acta Pharmacol Sin 37(12):1543–1554
Li J, Li L, Wang S, Zhang C, Zheng L, Jia Y, Xu M, Zhu T, Zhang Y, Rong R (2018) Resveratrol alleviates inflammatory responses and oxidative stress in rat kidney ischemia-reperfusion injury and H2O2-induced NRK-52E cells via the Nrf2/TLR4/NF-κB pathway. Cell Physiol Biochem 45(4):1677–1689
Norio H, Hiroshi W, Nobuhide A, Mitsue K, Jiro I, Yushiro T (1990) Cerebral ischemia model with conscious mice: involvement of NMDA receptor activation and derangement of learning and memory ability. J Pharmacol Methods 23(4):311–327
Schallert T, Kozlowski DA, Humm JL, Cocke RR (1997) Use-dependent structural events in recovery of function. Adv Neurol 73:229–238
Itoh J, Nabeshima T, Kameyama T (1990) Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacol 101(1):27–33
Morris R (1984) Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods 11(1):47–60
Shri R, Bora KS (2008) Neuroprotective effect of methanolic extracts of Allium cepa on ischemia and reperfusion-induced cerebral injury. Fitoterapia 79(2):86–96
Sheehan DC, Hrapchak BB (1980) Connective tissue and muscle fiber stains. Theory and practice of histotechnology 180–201
Ellman M (1959) A spectrophotometric method for determination of reduced glutathione in tissues. Anal Biochem 74:214–226
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247(10):3170–3175
Goth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–151
Grisham MB, Gaginella TS, von Ritter C, Tamai H, Robert MB, Granger DN (1990) Effects of neutrophil-derived oxidants on intestinal permeability, electrolyte transport, and epithelial cell viability. Inflammation 14(5):531–542
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Kahles T, Luedike P, Endres M, Galla HJ, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP (2007) NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke 38(11):3000–3006
Ponten U, Ratcheson RA, Salford LG, Siesjö BK (1973) Optimal freezing conditions for cerebral metabolites in rats. J Neurochem 21(5):1127–1138
Aşcı S, Demirci S, Aşcı H, Doğuç DK, Onaran İ (2016) Neuroprotective effects of pregabalin on cerebral ischemia and reperfusion. Balkan Med J 33(2):221
de Vries DK, Kortekaas KA, Tsikas D, Wijermars LG, van Noorden CJ, Suchy MT, Cobbaert CM, Klautz RJ, Schaapherder AF, Lindeman JH (2013) Oxidative damage in clinical ischemia/reperfusion injury: a reappraisal. Antioxid Redox Sign 19(6):535–545
Akhtar M, Pillai KK, Vohora D (2008) Effect of thioperamide on oxidative stress markers in middle cerebral artery occlusion model of focal cerebral ischemia in rats. Hum Exp Toxicol 27(10):761–767
Kim G, Kim E (2013) Effects of treadmill training on limb motor function and acetylcholinesterase activity in rats with stroke. J Phys Ther Sci 25(10):1227–1230
Milatovic D, Gupta RC, Aschner M (2006) Anticholinesterase toxicity and oxidative stress. The Sci World J 6:295–310
Melo JB, Agostinho P, Oliveira CR (2003) Involvement of oxidative stress in the enhancement of acetylcholinesterase activity induced by amyloid beta-peptide. Neurosci Res 45(1):117–127
Abdalla FH, Cardoso AM, Pereira LB, Schmatz R, Gonçalves JF, Stefanello N, Fiorenza AM, Gutierres JM, da Silva Serres JD, Zanini D, Pimentel VC (2013) Neuroprotective effect of quercetin in ectoenzymes and acetylcholinesterase activities in cerebral cortex synaptosomes of cadmium-exposed rats. Mol Cell Biochem 381(1):1–8
Daemen MA, Wolfs TG, Buurman WA (1999) Ischemia/reperfusion-induced IFN-γ up-regulation: involvement of IL-12 and IL-18. J Immunol 162(9):5506–5510
Khan H, Gupta A, Singh TG, Kaur A (2021) Mechanistic insight on the role of leukotriene receptors in ischemic–reperfusion injury. Pharmacol Rep 73:1240–1254
Chalouhi N, Jabbour P, Magnotta V, Hasan D (2014) Molecular imaging of cerebrovascular lesions. Transl Stroke Res 5(2):260–268
Román GC, Tatemichi TK, Erkinjuntti T, Cummings JL, Masdeu JC, Garcia JH, Amaducci L, Orgogozo JM, Brun A, Hofman A, Moody DM (1993) Vascular dementia: diagnostic criteria for research studies: report of the NINDS-AIREN International Workshop. J Neurol 43(2):250–250
Schimidt HL, Vieira A, Altermann C, Martins A, Sosa P, Santos FW, Mello-Carpes PB, Izquierdo I, Carpes FP (2014) Memory deficits and oxidative stress in cerebral ischemia–reperfusion: neuroprotective role of physical exercise and green tea supplementation. Neurobiol Learn Mem 114:242–250
Barrionuevo G, Brown TH (1983) Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci 80(23):7347–7351
Joshi CN, Jain SK, Murthy PS (2004) An optimized triphenyltetrazolium chloride method for identification of cerebral infarcts. Brain Res Prot 13(1):11–17
Bochelen D, Rudin M, Sauter A (1999) Calcineurin inhibitors FK506 and SDZ ASM 981 alleviate the outcome of focal cerebral ischemic/reperfusion injury. J Pharmacol Exp Ther 288(2):653–659
Moshfegh A, Setorki M (2017) Neuroprotective effect of matricaria chamomilla extract on motor dysfunction induced by transient global cerebral ischemia and reperfusion in rat Zahedan. J Res Med Sci. https://doi.org/10.5812/zjrms.10927
Grewal AK, Jaggi AS, Rana AC, Singh N (2013) Effect of neurosteroid modulation on global ischaemia-reperfusion-induced cerebral injury in mice. The Korean J Physiol & Pharmacol 17(6):485–491
Gulati P, Singh N (2014) Pharmacological evidence for connection of nitric oxide-mediated pathways in neuroprotective mechanism of ischemic postconditioning in mice. J Pharm Bioallied Sci 6(4):233
Reddy VD, Padmavathi P, Kavitha G, Saradamma B, Varadacharyulu N (2013) Alcohol-induced oxidative/nitrosative stress alters brain mitochondrial membrane properties. Mol Cell Biochem 375(1):39–47
Gaur V, Kumar A (2012) Effect of nonselective and selective COX-2 inhibitors on memory dysfunction, glutathione system, and tumor necrosis factor alpha level against cerebral ischemia reperfusion injury. Drug Chem Toxicol 35(2):218–224
Ozerol E, Bilgic S, Iraz M, Cigli A, Ilhan A, Akyol O (2009) The protective effect of erdosteine on short-term global brain ischemia/reperfusion injury in rats. Prog Neuropsychopharmacol Biol Psychiatry 33(1):20–24
Gupta R, Singh M, Sharma A (2003) Neuroprotective effect of antioxidants on ischaemia and reperfusion-induced cerebral injury. Pharmacol Res 48(2):209–215
Sanderson TH, Reynolds CA, Kumar R, Przyklenk K, Hüttemann M (2013) Molecular mechanisms of ischemia–reperfusion injury in brain: pivotal role of the mitochondrial membrane potential in reactive oxygen species generation. Mol Neurobiol 47(1):9–23
Zhao ZQ, Vinten-Johansen J (2006) Postconditioning: reduction of reperfusion-induced injury. Cardiovasc Res 70(2):200–211
Jordan JE, Zhao ZQ, Vinten-Johansen J (1999) The role of neutrophils in myocardial ischemia–reperfusion injury. Cardiovasc Res 43(4):860–878
Hearse DJ, Bolli R (1991) Reperfusion induced injury: manifestations, mechanisms, and clinical relevance. Trends Cardiovasc Med 26(2):101–108
Forman MB, Virmani R, Puett DW (1990) Mechanisms and therapy of myocardial reperfusion injury. Circulation 81(3):69–78
Becker LC, Ambrosio G (1987) Myocardial consequences of reperfusion. Prog Cardiovasc Dis 30(1):23–44
Zhao H, Ren C, Chen X, Shen J (2010) From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets 13(2):173–187
Ovize M, Baxter GF, Di Lisa F, Ferdinandy P, Garcia-Dorado D, Hausenloy DJ, Heusch G, Vinten-Johansen J, Yellon DM, Schulz R (2010) Postconditioning and protection from reperfusion injury: where do we stand? Position paper from the working group of cellular biology of the heart of the European society of cardiology. Cardiovasc Res 87(3):406–423
Andreadou I, Iliodromitis EK, Koufaki M, Kremastinos DT (2008) Pharmacological pre-and post-conditioning agents: reperfusion-injury of the heart revisited. Mini Rev Med Chem 8(9):952–959
Du DS, Ma XB, Zhang JF, Zhou XY, Li Y, Zhang YM, Qiao WL (2010) The protective effect of capsaicin receptor-mediated genistein postconditioning on gastric ischemia–reperfusion injury in rats. Dig Dis Sci 55(11):3070–3077
Goyal A, Kumar S, Nagpal M, Singh I, Arora S (2011) Potential of novel drug delivery systems for herbal drugs. Indian J Pharm Educ 45(3):225–235
Tong G, Sun Z, Wei X, Gu C, Kaye AD, Wang Y, Li J, Zhang Q, Guo H, Yu S, Yi D (2011) U50, 488H postconditioning reduces apoptosis after myocardial ischemia and reperfusion. J Life Sci 88(1–2):31–38
Das S, Cordis GA, Maulik N, Das DK (2005) Pharmacological preconditioning with resveratrol: role of CREB-dependent Bcl-2 signaling via adenosine A3 receptor activation. Am J Physiol Heart Circ Physiol 288(1):H328–H335
Li K, Gong X, Kuang G, Jiang R, Wan J, Wang B (2016) Sesamin protects against renal ischemia reperfusion injury by promoting CD39-adenosine-A2AR signal pathway in mice. Am J Transl Res 8(5):2245
Zhong Y, Zhu Y, He T, Li W, Yan H, Miao Y (2016) Rolipram-induced improvement of cognitive function correlates with changes in hippocampal CREB phosphorylation, BDNF and Arc protein levels. Neurosci Lett 610:171–176
Kwak HJ, Park KM, Choi HE, Chung KS, Lim HJ, Park HY (2008) PDE4 inhibitor, roflumilast protects cardiomyocytes against NO-induced apoptosis via activation of PKA and Epac dual pathways. Cell Signal 20(5):803–814
Chen J, Yu H, Zhong J, Feng H, Wang H, Cheng Y, Zou Z, Huang C, Zhou Z, Zheng W, Xu J (2018) The phosphodiesterase-4 inhibitor, FCPR16, attenuates ischemia-reperfusion injury in rats subjected to middle cerebral artery occlusion and reperfusion. Brain Res Bull 137:98–106
Acknowledgements
The authors are grateful to the Chitkara College of Pharmacy, Chitkara University, Punjab, India for providing the necessary facilities to carry out the research work.
Author information
Authors and Affiliations
Contributions
Amarjot Kaur: AK Thakur Gurjeet Singh: TGS, Heena Khan: HK, Manish Kumar: MN. Conceptualization: AK & TGS, Performed the experiments: AK & HK. Analyzed the data: TGS & MN, Review & editing: AK, NS, TGS & MMAD, Critically reviewed the article: TGS, All authors read and approved the final manuscript. All data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests. All authors read and approved the final manuscript.
Ethical Approval
The experimental protocol was conducted and approved as per the guidelines of the Institutional Animal Ethics Committee (IAEC) under registration number: 1181/PO/ReBi/S/08/CPCSEA and the guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA), Ministry of Environment and Forests, Government of India were followed.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kaur, A., Singh, T.G., Khan, H. et al. Neuroprotective Effect of Piclamilast-Induced Post-Ischemia Pharmacological Treatment in Mice. Neurochem Res 47, 2230–2243 (2022). https://doi.org/10.1007/s11064-022-03609-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03609-w