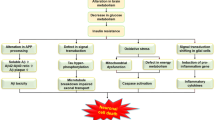
Abstract
Alzheimer’s disease (AD) and type 2 diabetes (T2D) major feature is insulin resistance. Brain and peripheral insulin resistance lead to hyperglycemia, which contributes to the development of T2D-linked comorbidities, such as obesity and dyslipidemia. Individuals with hyperglycemia in AD present with neuronal loss, formation of plaques and tangles and reduced neurogenesis. Inflammation seems to play an essential role in the development of insulin resistance in AD and T2D. We conducted a literature review about the links between AD and T2D. Alterations in glucose metabolism result from changes in the expression of the insulin receptor substrates 1 and 2 (IRS-1 and IRS-2), and seem to be mediated by several inflammatory pathways being present in both pathologies. Although there are some similarities in the insulin resistance of AD and T2D, brain and peripheral insulin resistance also have their discrete features. Failure to activate IRS-1 is the hallmark of AD, while inhibition of IRS-2 is the main feature in T2D. Inflammation mediates the alterations in glucose metabolism in AD and T2D. Targeting inflammation and insulin receptors may be a successful strategy to prevent and ameliorate T2D and AD symptoms.
Similar content being viewed by others
References
Alzheimer’s Association (2010) Alzheimer´s disease facts and figures. Alzheimer’s Assoc 13:1–74
Hölscher C (2014) First clinical data of the neuroprotective effects of nasal insulin application in patients with Alzheimer’s disease. Alzheimer’s Dement 10:33–37. https://doi.org/10.1016/j.jalz.2013.12.006
Ferreira S, Vieira M, De Felice F (2007) Soluble protein oligomers as emerging toxins in Alzheimer’s and other amyloid diseases. IUBMB Life 59:332–345. https://doi.org/10.1080/15216540701283882
Ferreira ST, Klein WL, Ferreira KWL (2011) The Aβ oligomer hypothesis for synapse failure and memory loss in Alzheimer’s disease. Neurobiol Learn Mem 96:529–543. https://doi.org/10.1016/j.nlm.2011.08.003
Lourenco MV, Clarke JR, Frozza RL et al (2013) TNF-α mediates PKR-dependent memory impairment and brain IRS-1 inhibition induced by Alzheimer’s β-amyloid oligomers in mice and monkeys. Cell Metab 18:831–843. https://doi.org/10.1016/j.cmet.2013.11.002
Ferreira ST, Clarke JR, Bomfim TR, De Felice FG (2014) Inflammation, defective insulin signaling, and neuronal dysfunction in Alzheimer’s disease. Alzheimer’s Dement 10:S76–S83. https://doi.org/10.1016/j.jalz.2013.12.010
Finch CE, Morgan TE (2003) Inflammatory processes of Alzheimer disease and aging. Proc Indian natn Sci Acad 178:165–177
Mandelkow E, Mandelkow E (1998) Tau in Alzheimer’s disease. Trends Cell Biol 8:425–427. https://doi.org/10.1016/S0962-8924(98)01368-3
Bomfim TR, Forny-germano L, Sathler LB et al (2012) An anti-diabetes agent protects the mouse brain from defective insulin signaling caused by Alzheimer´s disease-associated Aβ oligomers. J Clin Invest 122:1339–1353. https://doi.org/10.1172/JCI57256DS1
Morgen K, Frölich L (2015) The metabolism hypothesis of Alzheimer’s disease: from the concept of central insulin resistance and associated consequences to insulin therapy. J Neural Transm 122:499–504. https://doi.org/10.1007/s00702-015-1377-5
Abbott M, Wells DG, Fallon JR (1999) The insulin receptor tyrosine kinase substrate p58/53 and the insulin receptor are components of CNS synapses. J Neurosci 19:7300–7308
Kern W, Fruehwald-schultes B, Deininger E et al (2001) Improving Influence of Insulin on cognitive function in humans. Clin Neuroendocrinol 74:270–280
Craft S, Newcomer J, Kanne S et al (1996) Memory improvement following induced hyperinsulinemia in Alzheimer’s disease. Neurobiol Aging 17:123–130
Chiu S-L, Chen C-M, Cline HT (2011) Insulin receptor signaling regulates synapse number, dendritic plasticity and circuit function in vivo. Neuron 58:708–719. https://doi.org/10.1016/j.neuron.2008.04.014.Insulin
Brito-Moreira J, Lourenco MV, Oliveira MM et al (2017) Interaction of amyloid-β (Aβ) oligomers with neurexin 2α and neuroligin 1 mediates synapse damage and memory loss in mice. J Biol Chem 292:7327–7337. https://doi.org/10.1074/jbc.M116.761189
American Diabetes Association (ADA) (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(1):S81–90. https://doi.org/10.2337/dc14-S081
Sousa RAL, Freitas DA, Leite HR (2019) Cross-talk between obesity and central nervous system: role in cognitive function. Interv Obes Diabetes 3:7–9. https://doi.org/10.31031/IOD.2019.03.000551
De Sousa RAL, de Lima EV, da Silva TP et al (2019) Late cognitive consequences of gestational diabetes to the offspring, in a new mouse model. Mol Neurobiol 56:1–11. https://doi.org/10.1007/s12035-019-1624-0
Wang F, Shang Y, Zhang R et al (2018) A SIRT1 agonist reduces cognitive decline in type 2 diabetic rats through antioxidative and anti-inflammatory mechanisms. Mol Med Rep 1–9: https://doi.org/10.3892/mmr.2018.9699
Snel M, Gastaldelli A, Ouwens DM et al (2012) Effects of adding exercise to a 16-week very low-calorie diet in obese, insulin-dependent type 2 diabetes mellitus patients. J Clin Endocrinol Metab 97:2512–2520. https://doi.org/10.1210/jc.2011-3178
Cartee GD, Hepple RT, Bamman MM, Zierath JR (2016) Exercise Promotes Healthy Aging of Skeletal Muscle. Cell Metab 23:1034–1047. https://doi.org/10.1016/j.cmet.2016.05.007
IDF (2015) International Diabetes Federation, 7th edn. Diabetes Atlas, Brussels
da Marques N, SF, Abreu LC de, Santos BV dos, et al (2018) Cardiorespiratory parameters and glycated hemoglobin of patients with type 2 diabetes after a rehabilitation program. Medicine (Baltimore) 97:e9321. https://doi.org/10.1097/MD.0000000000009321
Stoeckli R, Keller U (2004) Nutritional fats and the risk of type 2 diabetes and cancer. Physiol Behav 83:611–615. https://doi.org/10.1016/j.physbeh.2004.07.030
American Diabetes Association (ADA) (2014) Standards of medical care in diabetes–2014. Diabetes Care 37(1):S14–80. https://doi.org/10.2337/dc14-S014
Sousa RAL de (2017) Brief report of the effects of the aerobic, resistance, and high-intensity interval training in type 2 diabetes mellitus individuals Diabetes mellitus. Int J Diabetes Dev Ctries. https://doi.org/10.1007/s13410-017-0582-1
Folli F, Saad MJ, Backer JM, Kahn CR (1992) Insulin stimulation of phosphatidylinositol 3-kinase activity and association with insulin receptor substrate 1 in liver and muscle of the intact rat. J Biol Chem 267:22171–22177
McGlone ER, Tan TM (2018) Of mice not men? Actions of interleukin-6 on glucose tolerance. Cell Metab 27:1157–1158. https://doi.org/10.1016/j.cmet.2018.05.013
Lang Lehrskov L, Lyngbaek MP, Soederlund L et al (2018) Interleukin-6 delays gastric emptying in humans with direct effects on glycemic control. Cell Metab 27:1201–1211.e3. https://doi.org/10.1016/j.cmet.2018.04.008
de Sousa RAL, Pardono E (2014) Report on the resistance to insulin and the benefits of intense exercise in diabetes type 2. Saúde e Pesqui 7:335–340
Carvalho CRO, Carvalheira JBC, Lima MHM et al (2003) Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology 144:638–647. https://doi.org/10.1210/en.2002-220706
Cai D, Dhe-Paganon S, Melendez P, a, et al (2003) Two new substrates in insulin signaling, IRS5/DOK4 and IRS6/DOK5. J Biol Chem 278:25323–25330. https://doi.org/10.1074/jbc.M212430200
Sun XJ, Rothenberg P, Kahn R et al (1991) Structure of the insulin receptor substrate IRS-1 defines a unique signal transduction protein. Nature 352:73–77
Sun XJ, Wang L, Zhang Y et al (1995) Role of IRS-2 in insulin and cytokine signalling. Nature 377:173–177
Copps KD, White MF (2014) Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 55:2565–2582. https://doi.org/10.1007/s00125-012-2644-8.Regulation
Carvalho CR, Brenelli SL, Silva AC et al (1996) Effect of aging on insulin receptor, insulin receptor substrate-1, and phosphatidylinositol 3-kinase in liver and muscle of rats. Endocrinology 137:151–159. https://doi.org/10.1210/endo.137.1.8536607
Lavan BE, Lienhard GE (1993) The insulin-elicited 60-kDa phosphotyrosine protein in rat adipocytes is associated with phosphatidylinositol 3-kinase. J Biol Chem 268:5921–5928
Fantin VR, Wang Q, Lienhard GE et al (2000) Mice lacking insulin receptor substrate 4 exhibit mild defects in growth, reproduction, and glucose homeostasis. Am J Physiol Endocrinol Metab 278:127–133
Withers DJ, Gutierrez JS, Towery H et al (1998) Disruption of IRS-2 causes type 2 diabetes in mice. Nature 391:900–904
Saad MJA, Araki E, Rothenberg PL et al (1992) Regulation of insulin receptor substrate-1 in liver and muscle of animal models of insulin resistance. J Clin Invest 90:1839–1849
Röhling M, Herder C, Stemper T, Müssig K (2016) Influence of acute and chronic exercise on glucose uptake. J Diabetes Res 2016:1–33. https://doi.org/10.1155/2016/2868652
Huang C, Thirone ACP, Huang X, Klip A (2005) Differential contribution of insulin receptor substrates 1 versus 2 to insulin signaling and glucose uptake in l6 myotubes. J Biol Chem 280:19426–19435. https://doi.org/10.1074/jbc.M412317200
Zierath JR (2002) Invited review: exercise training-induced changes in insulin signaling in skeletal muscle. J Appl Physiol 93:773–781. https://doi.org/10.1152/japplphysiol.00126.2002
Czech MP, Corvera S (1999) Signaling mechanisms that regulate glucose transport. J Biol Chem 274:1865–1868. https://doi.org/10.1074/jbc.274.4.1865
Backer JM Jr, Myer GM, Shoelson SE et al (1992) Phosphatidylinositol 3’-kinase is activated by association with IRS-1 during insulin stimulation. EMBO J 11:3469–3479
Ettcheto M, Busquets O, Camins A (2019) Potential preventive disease-modifying pharmacological strategies to delay late onset Alzheimer’s disease. Neural Regen Res 14:1721–1725. https://doi.org/10.4103/1673-5374.257513
Figueiredo CP, Clarke JR, Ledo JH et al (2013) Memantine rescues transient cognitive impairment caused by high-molecular-weight aβ oligomers but not the persistent impairment induced by low-molecular-weight oligomers. J Neurosci 33:9626–9634. https://doi.org/10.1523/JNEUROSCI.0482-13.2013
Ettcheto M, Sánchez-López E, Gómez-Mínguez Y et al (2018) Peripheral and central effects of memantine in a mixed preclinical mice model of obesity and familial Alzheimer’s disease. Mol Neurobiol 55:7327–7339. https://doi.org/10.1007/s12035-018-0868-4
Mucke L, Masliah E, Yu G et al (2000) High-level neuronal expression of Aβ 1–42 in wild-type human amyloid protein precursor transgenic mice : synaptotoxicity without plaque formation. J Neurosci 20:4050–4058
Glenner GG, Wong CW (1984) Alzheimer´s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 120:885–890
Koo EH, Sisodia SS, Archert DR et al (1990) Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Neurobiology 87:1561–1565
Tanzi RE, Haines JL, Watkins PC et al (1988) Genetic linkage map of human chromosome. Genomics 136:129–136
Ferreira T, Gralle M (2007) Structure and functions of the human amyloid precursor protein: the whole is more than the sum of its parts. Prog Neurobiol 82:11–32. https://doi.org/10.1016/j.pneurobio.2007.02.001
Vassar R, Kovacs DM, Yan R, Wong PC (2010) The beta-secretase enzyme BACE in health and Alzheimer´s disease: regualation, cell biology, function, and therapeutic potential. Neuroscience 29:12787–12794. https://doi.org/10.1523/JNEUROSCI.3657-09.2009.The
Bertram L, Lill CM, Tanzi RE (2010) Review the genetics of Alzheimer disease: back to the future. Neuron 68:270–281. https://doi.org/10.1016/j.neuron.2010.10.013
De SB, Vassar R, Golde T (2010) The secretases: enzymes with therapeutic potetntial in Alzheimer disease. Nat Rev Neurol 6:99–107. https://doi.org/10.1038/nrneurol.2009.218.The
Lacor PN, Buniel MC, Chang L et al (2004) Synaptic targeting by Alzheimer’s-related amyloid beta oligomers. J Neurosci 24:10191–10200. https://doi.org/10.1523/JNEUROSCI.3432-04.2004
Lee C-C, Kuo Y-M, Huang C-C, Hsu K-S (2009) Insulin rescues amyloid beta-induced impairment of hippocampal long-term potentiation. Neurobiol Aging 30:377–387. https://doi.org/10.1016/j.neurobiolaging.2007.06.014
Shankar GM, Li S, Mehta TH et al (2008) Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med 14:837–842. https://doi.org/10.1038/nm1782
Escribano L, Simón A-M, Gimeno E et al (2010) Rosiglitazone rescues memory impairment in Alzheimer’s transgenic mice: mechanisms involving a reduced amyloid and tau pathology. Neuropsychopharmacology 35:1593–1604. https://doi.org/10.1038/npp.2010.32
Lourenco MV, Frozza RL, de Freitas GB et al (2019) Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer’s models. Nat Med. https://doi.org/10.1038/s41591-018-0275-4
Batista AF, Frony-Germano L, Clarke JR et al (2018) The diabetes drug liraglutide reverses cognitive impairment in mice and attenuates insulin receptor and synaptic pathology in a non-human primate model of Alzheimer´s disease. J Pathol 245:85–100. https://doi.org/10.1002/path.5056
Borchelt DR, Thinakaran G, Eckman CB et al (1996) Familial Alzheimer’s disease – linked presenilin 1 variants elevate Aβ 1–42/1–40 ratio in vitro and in vivo. Neuron 17:1005–1013
Schmechel D, Saunders A, Strittmatter W et al (1993) Increased amyloid,8-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Neurobiology 90:9649–9653
Strittmatter WJ, Saunders ANNM, Schmechel D et al (1993) Apolipoprotein E: high-avidity binding to, B-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease 4°C. Med Sci 90:1977–1981
Soto C, Estrada L, Castilla J (2006) Amyloids, prions and the inherent infectious nature of misfolded protein aggregates. Trends Biochem Sci 31:150–155. https://doi.org/10.1016/j.tibs.2006.01.002
Gleckman AM, Evans RJ, Bell MD et al (2000) Optic nerve damage in shaken baby syndrome detection by β-amyloid precursor protein immunohistochemistry. Arch Pathol Lab Med 124:251–256
Hogan-Cann AD, Anderson CM (2016) Physiological roles of non-neuronal NMDA receptors. Trends Pharmacol Sci 37:750–767. https://doi.org/10.1016/j.tips.2016.05.012
Zhao W-Q, Santini F, Breese R et al (2010) Inhibition of calcineurin-mediated endocytosis and alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors prevents amyloid beta oligomer-induced synaptic disruption. J Biol Chem 285:7619–7632. https://doi.org/10.1074/jbc.M109.057182
Lacor PN, Buniel MC, Furlow PW et al (2007) Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in Alzheimer’s disease. J Neurosci 27:796–807. https://doi.org/10.1523/JNEUROSCI.3501-06.2007
Magdesian MH, Nery AA, Martins AHB et al (2005) Peptide blockers of the inhibition of neuronal nicotinic acetylcholine receptors by amyloid beta. J Biol Chem 280:31085–31090. https://doi.org/10.1074/jbc.M502406200
Magdesian MH, Carvalho MMVF, Mendes FA et al (2008) Amyloid-beta binds to the extracellular cysteine-rich domain of Frizzled and inhibits Wnt/beta-catenin signaling. J Biol Chem 283:9359–9368. https://doi.org/10.1074/jbc.M707108200
Sturchler E, Galichet A, Weibel M et al (2008) Site-specific blockade of RAGE-Vd prevents amyloid-beta oligomer neurotoxicity. J Neurosci 28:5149–5158. https://doi.org/10.1523/JNEUROSCI.4878-07.2008
Knowles JK, Rajadas J, Nguyen T-VV et al (2009) The p75 neurotrophin receptor promotes amyloid-beta(1–42)-induced neuritic dystrophy in vitro and in vivo. J Neurosci 29:10627–10637. https://doi.org/10.1523/JNEUROSCI.0620-09.2009
Neves FS, Marques PT, Aragão FB et al (2016) Brain-defective insulin signaling is associated to late cognitive impairment in post-septic mice. Mol Neurobiol. https://doi.org/10.1007/s12035-016-0307-3
De Felice FG, Vieira M, Bomfim T et al (2009) Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc Natl Acad Sci USA 106:1971–1976. https://doi.org/10.1073/pnas.0809158106
Hotamisligil GS, Peraldi P, Budavari A et al (1996) IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-ac- and obesity-induced insulin resistance. Science 80(271):665–668
Boden G, Shulman GI (2002) Free fatty acids in obesity and type 2 diabetes : defining their role in the development of insulin resistance and β -cell dysfunction. Eur J Clin Invest 32:14–23
Hirosumi J, Tuncman G, Chang L et al (2002) A central role for JNK in obesity and insulin resistance. Nature 2:10–13
Gregor MF, Yang L, Fabbrini E et al (2009) Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes 58:693–700. https://doi.org/10.2337/db08-1220.M.F.G
Arnold SE, Lucki I, Brookshire BR et al (2014) High fat diet produces brain insulin resistance, synaptodendritic abnormalities and altered behavior in mice. Neurobiol Dis 67:79–87. https://doi.org/10.1016/j.nbd.2014.03.011
de la Monte SM (2017) Insulin resistance and neurodegeneration: progress towards the development of new therapeutics for Alzheimer’s disease. Drugs 77:47–65. https://doi.org/10.1007/s40265-016-0674-0
Maurer MH, Geomor HK, Bürgers HF et al (2006) Adult neural stem cells express glucose transporters GLUT1 and GLUT3 and regulate GLUT3 expression. FEBS Lett 580:4430–4434. https://doi.org/10.1016/j.febslet.2006.07.012
Pratchayasakul W, Kerdphoo S, Petsophonsakul P et al (2011) Effects of high-fat diet on insulin receptor function in rat hippocampus and the level of neuronal corticosterone. Life Sci 88:619–627. https://doi.org/10.1016/j.lfs.2011.02.003
Nichols MR, St-Pierre M-K, Wendeln A-C et al (2019) Inflammatory mechanisms in neurodegeneration. J Neurochem. https://doi.org/10.1111/jnc.14674
Chen Z, Trapp BD (2016) Microglia and neuroprotection. J Neurochem 136(Suppl):10–17. https://doi.org/10.1111/jnc.13062
Bhat R, Crowe EP, Bitto A et al (2012) Astrocyte senescence as a component of Alzheimer’s disease. PLoS ONE 7:1–10. https://doi.org/10.1371/journal.pone.0045069
Busquets O, Ettcheto M, Eritja À et al (2019) c-Jun N-terminal Kinase 1 ablation protects against metabolic-induced hippocampal cognitive impairments. J Mol Med 97:1723–1733. https://doi.org/10.1007/s00109-019-01856-z
Gonçalves RA, Wijesekara N, Fraser PE, De Felice FG (2019) The link between tau and insulin signaling: implications for Alzheimer’s disease and other tauopathies. Front Cell Neurosci 13:1–7. https://doi.org/10.3389/fncel.2019.00017
Folch J, Olloquequi J, Ettcheto M et al (2019) The involvement of peripheral and brain insulin resistance in late onset Alzheimer’s dementia. Front Aging Neurosci 11:1–16. https://doi.org/10.3389/fnagi.2019.00236
Folch J, Ettcheto M, Busquets O et al (2018) The implication of the brain insulin receptor in late onset Alzheimer’s disease dementia. Pharmaceuticals 11:1–16. https://doi.org/10.3390/ph11010011
Grillo CA, Piroli GG, Lawrence RC et al (2015) Hippocampal insulin resistance impairs spatial learning and synaptic plasticity. Diabetes 64:3927–3936. https://doi.org/10.2337/db15-0596
Ferrario CR, Reagan LP (2018) Insulin-mediated synaptic plasticity in the CNS: anatomical, functional and temporal contexts. Physiol Behav 136:182–191. https://doi.org/10.1016/j.physbeh.2017.03.040
De Sousa RAL, Improta-caria AC, De J-S et al (2020) High-intensity resistance training induces changes in cognitive function, but not in locomotor activity or anxious behavior in rats induced to type 2 diabetes. Physiol Behav 223:1–7. https://doi.org/10.1016/j.physbeh.2020.112998
Plum L, Schubert M, Brüning JC, Bru JC (2005) The role of insulin receptor signaling in the brain. Trends Endocrinol Metab 16:59–65. https://doi.org/10.1016/j.tem.2005.01.008
Zhao W, Alkon DL (2001) Role of insulin and insulin receptor in learning and memory. Mol Cell Endocrinol 177:125–134
Chiu SL, Cline HT (2010) Insulin receptor signaling in the development of neuronal structure and function. Neural Dev 5:1–18. https://doi.org/10.1186/1749-8104-5-7
Costello DA, Claret M, Al-Qassab H et al (2012) Brain deletion of insulin receptor substrate 2 disrupts hippocampal synaptic plasticity and metaplasticity. PLoS ONE 7:30–34. https://doi.org/10.1371/journal.pone.0031124
Gralle M (2017) The neuronal insulin receptor in its environment. J Neurochem 140:359–367. https://doi.org/10.1111/jnc.13909
Boyd FT, Clarke DW, Muther TF, Raizada MK (1985) Insulin receptors and insulin modulation of norepinephrine uptake in neuronal cultures from rat brain. J Biol Chem 260:15880–15884
Sauter A, Goldstein M, Engel J, Ueta K (1983) Effect of insulin on central catecholamines. Brain Res 260:330–333. https://doi.org/10.1016/0006-8993(83)90691-1
Könner AC, Hess S, Tovar S et al (2011) Role for insulin signaling in catecholaminergic neurons in control of energy homeostasis. Cell Metab 13:720–728. https://doi.org/10.1016/j.cmet.2011.03.021
Kleinridders A, Cai W, Cappellucci L et al (2015) Insulin resistance in brain alters dopamine turnover and causes behavioral disorders. Proc Natl Acad Sci USA 112:3463–3468. https://doi.org/10.1073/pnas.1500877112
O’Malley D, Shanley LJ, Harvey J (2003) Insulin inhibits rat hippocampal neurones via activation of ATP-sensitive K+ and large conductance Ca2+-activated K+ channels. Neuropharmacology 44:855–863. https://doi.org/10.1016/S0028-3908(03)00081-9
Hannaoui S, Shim SY, Cheng YC et al (2014) Cholesterol balance in prion diseases and Alzheimer’s disease. Viruses 6:4505–4535. https://doi.org/10.3390/v6114505
Rojek A, Niedziela M (2010) Insulin receptor and its relationship with different forms of insulin resistance. Adv Cell Biol 1:1–32. https://doi.org/10.2478/v10052-010-0004-8
Sullivan PW, Ghushchyan V, Ben-Joseph RH (2008) The effect of obesity and cardiometabolic risk factors on expenditures and productivity in the United States. Obesity (Silver Spring) 16:2155–2162. https://doi.org/10.1038/oby.2008.325
Saltiel AR, Kahn CR (2001) Insulin signalling and the regulation of glucose and lipid metabolism. Nature 414:799–806
Exalto LG, Whitmer RA, Kappele LJ, Biessels GJ (2012) An update on type 2 diabetes, vascular dementia and Alzheimer’s disease. Exp Gerontol 47:858–864. https://doi.org/10.1016/j.exger.2012.07.014
Kwok MK, Lin SL, Schooling CM (2018) Re-thinking Alzheimer’s disease therapeutic targets using gene-based tests. EBioMedicine. https://doi.org/10.1016/j.ebiom.2018.10.001
Egan MF, Kost J, Tariot PN et al (2018) Randomized trial of verubecestat for mild-to-moderate Alzheimer’s disease. N Engl J Med 378:1691–1703. https://doi.org/10.1056/NEJMoa1706441
Panza F, Lozupone M, Solfrizzi V et al (2018) BACE inhibitors in clinical development for the treatment of Alzheimer’s disease. Expert Rev Neurother 18:847–857. https://doi.org/10.1080/14737175.2018.1531706
Hawkes N (2017) Merck ends trial of potential Alzheimer’s drug verubecestat. BMJ 356:j845. https://doi.org/10.1136/bmj.j845
Gylys KH, Fein JA, Yang F et al (2004) Synaptic changes in Alzheimer’s disease accompanied by decreased PSD-95 fluorescence. Neurobiology 165:1809–1817
Saad MJA, Folli F, Kahn JA, Kahn CR (1993) Rapid publication. J Clin Invest 92:2065–2072
De Felice FG, Ferreira ST (2014) Inflammation, defective insulin signaling, and mitochondrial dysfunction as common molecular denominators connecting type 2 diabetes to Alzheimer disease. Diabetes 63:2262–2272. https://doi.org/10.2337/db13-1954
Fealy CE, Nieuwoudt S, Foucher JA et al (2018) Functional high intensity exercise training ameliorates insulin resistance and cardiometabolic risk factors in type 2 diabetes. Exp Physio. https://doi.org/10.1113/EP086844
Bordier L, Doucet J, Boudet J, Bauduceau B (2014) Update on cognitive decline and dementia in elderly patients with diabetes. Diabetes Metab 40:331–337. https://doi.org/10.1016/j.diabet.2014.02.002
Acknowledgements
We are thankful to Coordenação de Pessoal de Ensino Superior (CAPES) and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG). We also thank to the CIPq-Saúde of UFVJM for the physical support to the development of this study.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Contributions
RALS conceived the review and performed the literature search; RALS wrote the manuscript, with contributions from ARH; RALS, ARH, DAF, VAM, ACRL, HRL analyzed and critically discussed the data and reviewed the manuscript; RALS and HRL supervised the review. All authors have read and approved the final version of the manuscript, and agree with the order of presentation of the authors.
Corresponding author
Ethics declarations
Conflicts of interests
The authors declare that they have no conflict of interest. Financial or otherwise, are declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
De Sousa, R.A.L., Harmer, A.R., Freitas, D.A. et al. An update on potential links between type 2 diabetes mellitus and Alzheimer’s disease. Mol Biol Rep 47, 6347–6356 (2020). https://doi.org/10.1007/s11033-020-05693-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05693-z