Abstract
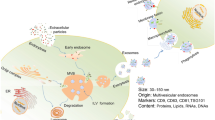
Posterior ocular disease, a disease that accounts for 55% of all ocular diseases, can contribute to permanent vision loss if left without treatment. Due to the special structure of the eye, various obstacles make it difficult for drugs to reach lesions in the posterior ocular segment. Therefore, the development of highly permeable targeted drugs and delivery systems is particularly important. Exosomes are a class of extracellular vesicles at 30–150 nm, which are secreted by various cells, tissues, and body fluids. They carry various signaling molecules, thus endowing them with certain physiological functions. In this review, we describe the ocular barriers and the biogenesis, isolation, and engineering of exosomes, as exosomes not only have pharmacological effects but also are good nanocarriers with targeted properties. Moreover, their biocompatibility and immunogenicity are better than synthetic nanocarriers. Most importantly, they may have the ability to pass through the blood–eye barrier. Thus, they may be developed as both targeted nano-drugs and nano-delivery vehicles for the treatment of posterior ocular diseases. We focus on the current status and potential application of exosomes as targeted nano-drugs and nano-delivery vehicles in posterior ocular diseases.



Similar content being viewed by others
Data availability
No data was used for the research described in the article.
References
Juliana FR, Kesse S, Boakye-Yiadom KO, Veroniaina H, Wang HH, Sun MH (2019) Promising approach in the treatment of glaucoma using nanotechnology and nanomedicine-based systems. Molecules. https://doi.org/10.3390/molecules24203805
Joseph RR, Venkatraman SS (2017) Drug delivery to the eye: what benefits do nanocarriers offer? Nanomedicine 12:683–702. https://doi.org/10.2217/nnm-2016-0379
Nayak K, Misra M (2018) A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother 107:1564–1582. https://doi.org/10.1016/j.biopha.2018.08.138
Wang R, Gao Y, Liu AC, Zhai GX (2021) A review of nanocarrier-mediated drug delivery systems for posterior segment eye disease: challenges analysis and recent advances. J Drug Target 29:687–702. https://doi.org/10.1080/1061186x.2021.1878366
Naidorf-Rosenblatt H, Landau-Part D, Moisseiev J, Alhalel A, Huna-Baron R, Skaat A, Pilus S, Levi L, Leshno A (2022) Ocular surface temperature differences in retinal vascular diseases. Retina-J Retinal Vitreous Dis 42:152–158. https://doi.org/10.1097/IAE.0000000000003278
Abdulla D, Ali Y, Menezo V, Taylor SRJ (2022) The use of sustained release intravitreal steroid implants in non-infectious uveitis affecting the posterior segment of the eye. Ophthalmol Therapy 11:479–487. https://doi.org/10.1007/s40123-022-00456-4
Moisseiev E, Loewenstein A (2017) Drug delivery to the posterior segment of the eye. Dev Ophthalmol 58:87–101. https://doi.org/10.1159/000455276
Weng YH, Liu J, Jin SB, Guo WS, Liang XJ, Hua ZB (2017) Nanotechnology-based strategies for treatment of ocular disease. Acta Pharma Sinica B 7:281–291. https://doi.org/10.1016/j.apsb.2016.09.001
del Amo EM, Rimpela AK, Heikkinen E, Kari OK, Ramsay E, Lajunen T, Schmitt M, Pelkonen L, Bhattacharya M, Richardson D, Subrizi A, Turunen T, Reinisalo M, Itkonen J, Toropainen E, Casteleijn M, Kidron H, Antopolsky M, Vellonen KS, Ruponen M, Urtti A (2017) Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res 57:134–185. https://doi.org/10.1016/j.preteyeres.2016.12.001
Yasin MN, Svirskis D, Seyfoddin A, Rupenthal ID (2014) Implants for drug delivery to the posterior segment of the eye: a focus on stimuli-responsive and tunable release systems. J Control Release 196:208–221. https://doi.org/10.1016/j.jconrel.2014.09.030
Qamar Z, Qizilbash FF, Iqubal MK, Ali A, Narang JK, Ali J, Baboota S (2019) Nano-based drug delivery system: recent strategies for the treatment of ocular disease and future perspective. Recent Pat Drug Deliv Formul 13:246–254. https://doi.org/10.2174/1872211314666191224115211
Srinivasarao DA, Lohiya G, Katti DS (2019) Fundamentals, challenges, and nanomedicine-based solutions for ocular diseases. Wiley Interdiscip Rev-Nanomed Nanobiotechnol. https://doi.org/10.1002/wnan.1548
Xu TT, Xu XY, Gu Y, Fang L, Cao F (2018) Functional intercalated nanocomposites with chitosan-glutathione-glycylsarcosine and layered double hydroxides for topical ocular drug delivery. Int J Nanomed 13:917–937. https://doi.org/10.2147/ijn.S148104
Bonilla L, Espina M, Severino P, Cano A, Ettcheto M, Camins A, Garcia ML, Souto EB, Sanchez-Lopez E (2022) Lipid nanoparticles for the posterior eye segment. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14010090
Vashisht M, Rani P, Onteru SK, Singh D (2017) Curcumin encapsulated in milk exosomes resists human digestion and possesses enhanced intestinal permeability in vitro. Appl Biochem Biotechnol 183:993–1007. https://doi.org/10.1007/s12010-017-2478-4
van den Boorn JG, Schlee M, Coch C, Hartmann G (2011) SiRNA delivery with exosome nanoparticles. Nat Biotechnol 29:325–326. https://doi.org/10.1038/nbt.1830
Salvati A, Pitek AS, Monopoli MP, Prapainop K, Bombelli FB, Hristov DR, Kelly PM, Aberg C, Mahon E, Dawson KA (2013) Transferrin-functionalized nanoparticles lose their targeting capabilities when a biomolecule corona adsorbs on the surface. Nat Nanotechnol 8:137–143. https://doi.org/10.1038/nnano.2012.237
Stewart JM, Keselowsky BG (2017) Combinatorial drug delivery approaches for immunomodulation. Adv Drug Deliv Rev 114:161–174. https://doi.org/10.1016/j.addr.2017.05.013
Liao W, Du Y, Zhang CH, Pan FW, Yao Y, Zhang T, Peng Q (2019) Exosomes: the next generation of endogenous nanomaterials for advanced drug delivery and therapy. Acta Biomater 86:1–14. https://doi.org/10.1016/j.actbio.2018.12.045
Record M, Subra C, Silvente-Poirot S, Poirot M (2011) Exosomes as intercellular signalosomes and pharmacological effectors. Biochem Pharmacol 81:1171–1182. https://doi.org/10.1016/j.bcp.2011.02.011
Yu B, Li XR, Zhang XM (2020) Mesenchymal stem cell-derived extracellular vesicles as a new therapeutic strategy for ocular diseases. World J Stem Cells 12:178–187. https://doi.org/10.4252/wjsc.v12.i3.178
Harrell CR, Markovic BS, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V (2018) Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Adv Exp Med Biol 1089:47–57. https://doi.org/10.1007/5584_2018_219
Zhu XH, Badawi M, Pomeroy S, Sutaria DS, Xie ZL, Baek A, Jiang JM, Elgamal OA, Mo XK, La Perle K, Chalmers J, Schmittgen TD, Phelps MA (2017) Comprehensive toxicity and immunogenicity studies reveal minimal effects in mice following sustained dosing of extracellular vesicles derived from HEK293T cells. J Extracellular Vesicles. https://doi.org/10.1080/20013078.2017.1324730
Quah BJC, O’Neill HC (2005) The immunogenicity of dendritic cell-derived exosomes. Blood Cells Mol Dis 35:94–110. https://doi.org/10.1016/j.bcmd.2005.05.002
Zheng MN, Huang M, Ma XY, Chen HZ, Gao XL (2019) Harnessing exosomes for the development of brain drug delivery systems. Bioconjug Chem 30:994–1005. https://doi.org/10.1021/acs.bioconjchem.9b00085
Manfré L, Midiri M, Giuffré G, Mangiameli A, Cardella G, Ponte F, De Maria M, Lagalla R (1997) Blood-ocular barrier damage: use of contrast-enhanced MRI. Eur Radiol 7:110–114. https://doi.org/10.1007/s003300050121
Desalvo MK, Mayer N, Mayer F, Bainton RJ (2011) Physiologic and anatomic characterization of the brain surface glia barrier of drosophila. Glia 59:1322–1340. https://doi.org/10.1002/glia.21147
Cattelotte J, Andre P, Ouellet M, Bourasset F, Scherrmann JM, Cisternino S (2008) In situ mouse carotid perfusion model: glucose and cholesterol transport in the eye and brain. J Cereb Blood Flow Metab 28:1449–1459. https://doi.org/10.1038/jcbfm.2008.34
Huang CJ, Quinn D, Sadovsky Y, Suresh S, Hsia KJ (2017) Formation and size distribution of self-assembled vesicles. Proc Natl Acad Sci USA 114:2910–2915. https://doi.org/10.1073/pnas.1702065114
Amrite AC, Edelhauser HF, Singh SR, Kompella UB (2008) Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol Vis 14:150–160
Tsai CH, Wang PY, Lin IC, Huang H, Liu GS, Tseng CL (2018) Ocular drug delivery: role of degradable polymeric nanocarriers for ophthalmic application. Int J Mol Sci. https://doi.org/10.3390/ijms19092830
Boddu SH, Gupta H, Patel S (2014) Drug delivery to the back of the eye following topical administration: an update on research and patenting activity. Recent Pat Drug Deliv Formul 8:27–36. https://doi.org/10.2174/1872211308666140130093301
Glasgow BJ (2020) Evidence for phospholipids on the surface of human tears. Investig Ophthalmol Vis Sci. https://doi.org/10.1167/iovs.61.14.19
Fernandes AR, Sanchez-Lopez E, Santini A, Santos Td, Garcia ML, Silva AM, Souto EB (2021) Mono- and dicationic DABCO/quinuclidine composed nanomaterials for the loading of steroidal drug: 32 factorial design and physicochemical characterization. Nanomaterials 11:2758. https://doi.org/10.3390/nano11102758
Ruponen M, Urtti A (2015) Undefined role of mucus as a barrier in ocular drug delivery. Eur J Pharm Biopharm 96:442–446. https://doi.org/10.1016/j.ejpb.2015.02.032
Awwad S, Ahmed A, Sharma G, Heng JS, Khaw PT, Brocchini S, Lockwood A (2017) Principles of pharmacology in the eye. Br J Pharmacol 174:4205–4223. https://doi.org/10.1111/bph.14024
DelMonte DW, Kim T (2011) Anatomy and physiology of the cornea. J Cataract Refract Surg 37:588–598. https://doi.org/10.1016/j.jcrs.2010.12.037
Sridhar MS (2018) Anatomy of cornea and ocular surface. Indian J Ophthalmol 66:190–194. https://doi.org/10.4103/ijo.IJO_646_17
Zhang YQ, Zhang WJ, Liu W, Hu XJ, Zhou GD, Cui L, Cao Y (2008) Tissue engineering of corneal stromal layer with dermal fibroblasts: phenotypic and functional switch of differentiated cells in cornea. Tissue Eng Part A 14:295–303. https://doi.org/10.1089/tea.2007.0200
Makuloluwa AK, Hamill KJ, Rauz S, Bosworth L, Haneef A, Romano V, Williams RL, Dartt DA, Kaye SB (2021) The conjunctival extracellular matrix, related disorders and development of substrates for conjunctival restoration. Ocul Surf. https://doi.org/10.1016/j.jtos.2021.05.011
Gipson IK (2016) Goblet cells of the conjunctiva: a review of recent findings. Prog Retin Eye Res 54:49–63. https://doi.org/10.1016/j.preteyeres.2016.04.005
Forrester JV, Dick AD, McMenamin PG, Roberts F, Pearlman E (2016) The eye: basic sciences in practice, 4th edn. Elsevier
Wang YY, Xu XY, Gu Y, Cheng YJ, Cao F (2018) Recent advance of nanoparticle-based topical drug delivery to the posterior segment of the eye. Expert Opin Drug Deliv 15:687–701. https://doi.org/10.1080/17425247.2018.1496080
Chen W, Tan X, Chen X (2017) Anatomy and physiology of the crystalline lens. In: Liu Y (ed) Pediatric lens diseases. Springer, Singapore, pp 21–28
Augusteyn RC (2010) On the growth and internal structure of the human lens. Exp Eye Res 90:643–654. https://doi.org/10.1016/j.exer.2010.01.013
Atta G, Tempfer H, Kaser-Eichberger A, Guo YW, Schroedl F, Traweger A, Heindl LM (2020) The lymphangiogenic and hemangiogenic privilege of the human sclera. Ann Anatomy-Anatomischer Anzeiger. https://doi.org/10.1016/j.aanat.2020.151485
Mahaling B, Katti DS (2016) Physicochemical properties of core-shell type nanoparticles govern their spatiotemporal biodistribution in the eye. Nanomed-Nanotechnol Biol Med 12:2149–2160. https://doi.org/10.1016/j.nano.2016.05.017
Hancock SE, Wan CR, Fisher NE, Andino RV, Ciulla TA (2021) Biomechanics of suprachoroidal drug delivery: from benchtop to clinical investigation in ocular therapies. Expert Opin Drug Deliv 18:777–788. https://doi.org/10.1080/17425247.2021.1867532
Thrimawithana TR, Young S, Bunt CR, Green C, Alany RG (2011) Drug delivery to the posterior segment of the eye. Drug Discov Today 16:270–277. https://doi.org/10.1016/j.drudis.2010.12.004
Kim SH, Lutz RJ, Wang NS, Robinson MR (2007) Transport barriers in transscleral drug delivery for retinal diseases. Ophthalmic Res 39:244–254. https://doi.org/10.1159/000108117
Velagaleti PR, Buonarati MH (2014) Challenges and strategies in drug residue measurement (bioanalysis) of ocular tissues. Methods Pharmacol Toxicol. https://doi.org/10.1007/7653_2013_6
Grassiri B, Zambito Y, Bernkop-Schnurch A (2021) Strategies to prolong the residence time of drug delivery systems on ocular surface. Adv Colloid Interface Sci. https://doi.org/10.1016/j.cis.2020.102342
Coca-Prados M (2014) The blood-aqueous barrier in health and disease. J Glaucoma 23:S36–S38. https://doi.org/10.1097/ijg.0000000000000107
Lee J, Pelis RM (2016) drug transport by the blood-aqueous humor barrier of the eye. Drug Metab Dispos 44:1675–1681. https://doi.org/10.1124/dmd.116.069369
Yemanyi F, Bora K, Blomfield AK, Wang Z, Chen J (2021) Wnt signaling in inner blood-retinal barrier maintenance. Int J Mol Sci. https://doi.org/10.3390/ijms222111877
Spaide RF, Klancnik JM Jr, Cooney MJ (2015) Retinal vascular layers imaged by fluorescein angiography and optical coherence tomography angiography. JAMA Ophthalmol 133:45–50. https://doi.org/10.1001/jamaophthalmol.2014.3616
Pitkänen L, Ranta VP, Moilanen H, Urtti A (2005) Permeability of retinal pigment epithelium: effects of permeant molecular weight and lipophilicity. Invest Ophthalmol Vis Sci 46:641–646. https://doi.org/10.1167/iovs.04-1051
Xu HZ, Le YZ (2011) Significance of outer blood-retina barrier breakdown in diabetes and ischemia. Invest Ophthalmol Vis Sci 52:2160–2164. https://doi.org/10.1167/iovs.10-6518
Díaz-Coránguez M, Ramos C, Antonetti DA (2017) The inner blood-retinal barrier: cellular basis and development. Vision Res 139:123–137. https://doi.org/10.1016/j.visres.2017.05.009
Harrell CR, Simovic Markovic B, Fellabaum C, Arsenijevic A, Djonov V, Arsenijevic N, Volarevic V (2018) Therapeutic potential of mesenchymal stem cell-derived exosomes in the treatment of eye diseases. Adv Exp Med Biol 1089:47–57. https://doi.org/10.1007/5584_2018_219
Gurung S, Perocheau D, Touramanidou L, Baruteau J (2021) The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signal 19:47. https://doi.org/10.1186/s12964-021-00730-1
Mathieu M, Martin-Jaular L, Lavieu G, Théry C (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol 21:9–17. https://doi.org/10.1038/s41556-018-0250-9
Harding C, Heuser J, Stahl P (1983) Receptor-mediated endocytosis of transferrin and recycling of the transferrin receptor in rat reticulocytes. J Cell Biol 97:329–339. https://doi.org/10.1083/jcb.97.2.329
Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C (1987) Vesicle formation during reticulocyte maturation: association of plasma membrane activities with released vesicles (exosomes). J Biol Chem 262:9412–9420. https://doi.org/10.1016/S0021-9258(18)48095-7
Lin H, Chen H, Zhao X, Ding T, Wang Y, Chen Z, Tian Y, Zhang P, Shen Y (2022) Advances of exosomes in periodontitis treatment. J Transl Med 20:279. https://doi.org/10.1186/s12967-022-03487-4
Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH (2019) Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. https://doi.org/10.3390/cells8040307
Kalluri R, LeBleu VS (2020) The biology, function, and biomedical applications of exosomes. Science. https://doi.org/10.1126/science.aau6977
Hanson PI, Cashikar A (2012) Multivesicular body morphogenesis. Annu Rev Cell Dev Biol 28:337–362. https://doi.org/10.1146/annurev-cellbio-092910-154152
Skotland T, Sandvig K, Llorente A (2017) Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66:30–41. https://doi.org/10.1016/j.plipres.2017.03.001
Zhang Y, Liu Y, Liu H, Tang WH (2019) Exosomes: biogenesis, biologic function and clinical potential. Cell Biosci 9:19. https://doi.org/10.1186/s13578-019-0282-2
Colombo M, Raposo G, Théry C (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol 30:255–289. https://doi.org/10.1146/annurev-cellbio-101512-122326
Nasser MI, Masood M, Adlat S, Gang D, Zhu S, Li G, Li N, Chen J, Zhu P (2021) Mesenchymal stem cell-derived exosome microRNA as therapy for cardiac ischemic injury. Biomed Pharmacother 143:112118. https://doi.org/10.1016/j.biopha.2021.112118
Yang Y, Yuan L, Du X, Zhou K, Qin L, Wang L, Yang M, Wu M, Zheng Z, Xiang Y, Qu X, Liu H, Qin X, Liu C (2021) Involvement of epithelia-derived exosomes in chronic respiratory diseases. Biomed Pharmacother 143:112189. https://doi.org/10.1016/j.biopha.2021.112189
Ma D, Zhang QY, Rong HM, Zhai K, Tong ZH (2022) Proteomic profiling and functional analysis of B cell-derived exosomes upon pneumocystis infection. J Immunol Res 2022:5187166. https://doi.org/10.1155/2022/5187166
Chen SW, Zhu SQ, Pei X, Qiu BQ, Xiong D, Long X, Lin K, Lu F, Xu JJ, Wu YB (2021) Cancer cell-derived exosomal circUSP7 induces CD8(+) T cell dysfunction and anti-PD1 resistance by regulating the miR-934/SHP2 axis in NSCLC. Mol Cancer 20:144. https://doi.org/10.1186/s12943-021-01448-x
Wu X, Xu X, Xiang Y, Fan D, An Q, Yue G, Jin Z, Ding J, Hu Y, Du Q, Xu J, Xie R (2022) Exosome-mediated effects and applications in inflammatory diseases of the digestive system. Eur J Med Res 27:163. https://doi.org/10.1186/s40001-022-00792-y
Zhang X, Xu Y, Ma L, Yu K, Niu Y, Xu X, Shi Y, Guo S, Xue X, Wang Y, Qiu S, Cui J, Wang H, Tian X, Miao Y, Meng F, Qiao Y, Yu Y, Wang J (2022) Essential roles of exosome and circRNA_101093 on ferroptosis desensitization in lung adenocarcinoma. Cancer Commun 42:287–313. https://doi.org/10.1002/cac2.12275
Wang L, Wang J, Wang Z, Zhou J, Zhang Y (2021) Higher urine exosomal miR-193a is associated with a higher probability of primary focal segmental glomerulosclerosis and an increased risk of poor prognosis among children with nephrotic syndrome. Front Cell Dev Biol 9:727370. https://doi.org/10.3389/fcell.2021.727370
Bae IS, Kim SH (2021) Milk exosome-derived microRNA-2478 suppresses melanogenesis through the Akt-GSK3β pathway. Cells. https://doi.org/10.3390/cells10112848
Hofmann L, Abou Kors T, Ezić J, Niesler B, Röth R, Ludwig S, Laban S, Schuler PJ, Hoffmann TK, Brunner C, Medyany V, Theodoraki MN (2022) Comparison of plasma- and saliva-derived exosomal miRNA profiles reveals diagnostic potential in head and neck cancer. Front Cell Dev Biol 10:971596. https://doi.org/10.3389/fcell.2022.971596
Crenshaw BJ, Sims B, Matthews QL (2018) Biological function of exosomes as diagnostic markers and therapeutic delivery vehicles in carcinogenesis and infectious diseases. Nanomedicines. https://doi.org/10.5772/intechopen.80225
Yang H, Yang S, Shen H, Wu S, Ruan J, Lyu G (2021) Construction of the amniotic fluid-derived exosomal ceRNA network associated with ventricular septal defect. Genomics 113:4293–4302. https://doi.org/10.1016/j.ygeno.2021.11.003
Thakur A, Parra DC, Motallebnejad P, Brocchi M, Chen HJ (2022) Exosomes: small vesicles with big roles in cancer, vaccine development, and therapeutics. Bioact Mater 10:281–294. https://doi.org/10.1016/j.bioactmat.2021.08.029
Chen J, Li P, Zhang T, Xu Z, Huang X, Wang R, Du L (2021) Review on strategies and technologies for exosome isolation and purification. Front Bioeng Biotechnol 9:811971. https://doi.org/10.3389/fbioe.2021.811971
Lin S, Yu Z, Chen D, Wang Z, Miao J, Li Q, Zhang D, Song J, Cui D (2020) Progress in microfluidics-based exosome separation and detection technologies for diagnostic applications. Small 16:e1903916. https://doi.org/10.1002/smll.201903916
Zhang MD, Jin K, Gao L, Zhang ZK, Li F, Zhou FF, Zhang L (2018) Methods and technologies for exosome isolation and characterization. Small Methods. https://doi.org/10.1002/smtd.201800021
Cao F, Gao Y, Chu Q, Wu Q, Zhao L, Lan T, Zhao L (2019) Proteomics comparison of exosomes from serum and plasma between ultracentrifugation and polymer-based precipitation kit methods. Electrophoresis 40:3092–3098. https://doi.org/10.1002/elps.201900295
Zhang Y, Bi J, Huang J, Tang Y, Du S, Li P (2020) Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomed 15:6917–6934. https://doi.org/10.2147/ijn.S264498
Liu WZ, Ma ZJ, Kang XW (2022) Current status and outlook of advances in exosome isolation. Anal Bioanal Chem 414:7123–7141. https://doi.org/10.1007/s00216-022-04253-7
Wang J, Chen D, Ho EA (2021) Challenges in the development and establishment of exosome-based drug delivery systems. J Control Release 329:894–906. https://doi.org/10.1016/j.jconrel.2020.10.020
Luan X, Sansanaphongpricha K, Myers I, Chen H, Yuan H, Sun D (2017) Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol Sin 38:754–763. https://doi.org/10.1038/aps.2017.12
Weng Z, Zhang B, Wu C, Yu F, Han B, Li B, Li L (2021) Therapeutic roles of mesenchymal stem cell-derived extracellular vesicles in cancer. J Hematol Oncol 14:136. https://doi.org/10.1186/s13045-021-01141-y
Liang Y, Duan L, Lu J, Xia J (2021) Engineering exosomes for targeted drug delivery. Theranostics 11:3183–3195. https://doi.org/10.7150/thno.52570
Butreddy A, Kommineni N, Dudhipala N (2021) Exosomes as naturally occurring vehicles for delivery of biopharmaceuticals: insights from drug delivery to clinical perspectives. Nanomaterials. https://doi.org/10.3390/nano11061481
Fuhrmann G, Serio A, Mazo M, Nair R, Stevens MM (2015) Active loading into extracellular vesicles significantly improves the cellular uptake and photodynamic effect of porphyrins. J Control Release 205:35–44. https://doi.org/10.1016/j.jconrel.2014.11.029
Lin Y, Lu Y, Li X (2020) Biological characteristics of exosomes and genetically engineered exosomes for the targeted delivery of therapeutic agents. J Drug Target 28:129–141. https://doi.org/10.1080/1061186x.2019.1641508
Kučuk N, Primožič M, Knez Ž, Leitgeb M (2021) Exosomes engineering and their roles as therapy delivery tools, therapeutic targets, and biomarkers. Int J Mol Sci. https://doi.org/10.3390/ijms22179543
Salunkhe S, Dheeraj BM, Chitkara D, Mittal A (2020) Surface functionalization of exosomes for target-specific delivery and in vivo imaging & tracking: strategies and significance. J Control Release 326:599–614. https://doi.org/10.1016/j.jconrel.2020.07.042
Paskeh MDA, Entezari M, Mirzaei S, Zabolian A, Saleki H, Naghdi MJ, Sabet S, Khoshbakht MA, Hashemi M, Hushmandi K, Sethi G, Zarrabi A, Kumar AP, Tan SC, Papadakis M, Alexiou A, Islam MA, Mostafavi E, Ashrafizadeh M (2022) Emerging role of exosomes in cancer progression and tumor microenvironment remodeling. J Hematol Oncol 15:83. https://doi.org/10.1186/s13045-022-01305-4
Han C, Yang J, Sun J, Qin G (2022) Extracellular vesicles in cardiovascular disease: biological functions and therapeutic implications. Pharmacol Ther 233:108025. https://doi.org/10.1016/j.pharmthera.2021.108025
Sun J, Shen H, Shao L, Teng X, Chen Y, Liu X, Yang Z, Shen Z (2020) HIF-1α overexpression in mesenchymal stem cell-derived exosomes mediates cardioprotection in myocardial infarction by enhanced angiogenesis. Stem Cell Res Ther 11:373. https://doi.org/10.1186/s13287-020-01881-7
Cano A, Muñoz-Morales Á, Sánchez-López E, Ettcheto M, Souto EB, Camins A, Boada M, Ruíz A (2023) Exosomes-based nanomedicine for neurodegenerative diseases: current insights and future challenges. Pharmaceutics. https://doi.org/10.3390/pharmaceutics15010298
Fan L, Liu C, Chen X, Zheng L, Zou Y, Wen H, Guan P, Lu F, Luo Y, Tan G, Yu P, Chen D, Deng C, Sun Y, Zhou L, Ning C (2022) Exosomes-loaded electroconductive hydrogel synergistically promotes tissue repair after spinal cord injury via immunoregulation and enhancement of myelinated axon growth. Adv Sci 9:e2105586. https://doi.org/10.1002/advs.202105586
Song Y, Wang B, Zhu X, Hu J, Sun J, Xuan J, Ge Z (2021) Human umbilical cord blood-derived MSCs exosome attenuate myocardial injury by inhibiting ferroptosis in acute myocardial infarction mice. Cell Biol Toxicol 37:51–64. https://doi.org/10.1007/s10565-020-09530-8
Santos P, Almeida F (2021) Exosome-based vaccines: history, current state, and clinical trials. Front Immunol 12:711565. https://doi.org/10.3389/fimmu.2021.711565
Zhou T, He C, Lai P, Yang Z, Liu Y, Xu H, Lin X, Ni B, Ju R, Yi W, Liang L, Pei D, Egwuagu CE, Liu X (2022) miR-204-containing exosomes ameliorate GVHD-associated dry eye disease. Sci Adv 8:eabj9617. https://doi.org/10.1126/sciadv.abj9617
Mathew B, Torres LA, Gamboa Acha L, Tran S, Liu A, Patel R, Chennakesavalu M, Aneesh A, Huang CC, Feinstein DL, Mehraeen S, Ravindran S, Roth S (2021) Uptake and distribution of administered bone marrow mesenchymal stem cell extracellular vesicles in retina. Cells. https://doi.org/10.3390/cells10040730
Zhang F, Guo J, Zhang Z, Qian Y, Wang G, Duan M, Zhao H, Yang Z, Jiang X (2022) Mesenchymal stem cell-derived exosome: a tumor regulator and carrier for targeted tumor therapy. Cancer Lett 526:29–40. https://doi.org/10.1016/j.canlet.2021.11.015
van Lookeren CM, LeCouter J, Yaspan BL, Ye W (2014) Mechanisms of age-related macular degeneration and therapeutic opportunities. J Pathol 232:151–164. https://doi.org/10.1002/path.4266
Kauppinen A, Paterno JJ, Blasiak J, Salminen A, Kaarniranta K (2016) Inflammation and its role in age-related macular degeneration. Cell Mol Life Sci 73:1765–1786. https://doi.org/10.1007/s00018-016-2147-8
Hajrasouliha AR, Jiang G, Lu Q, Lu H, Kaplan HJ, Zhang HG, Shao H (2013) Exosomes from retinal astrocytes contain antiangiogenic components that inhibit laser-induced choroidal neovascularization. J Biol Chem 288:28058–28067. https://doi.org/10.1074/jbc.M113.470765
Liu Z, Mao X, Yang Q, Zhang X, Xu J, Ma Q, Zhou Y, Da Q, Cai Y, Sopeyin A, Dong Z, Hong M, Caldwell RB, Sodhi A, Huo Y (2022) Suppression of myeloid PFKFB3-driven glycolysis protects mice from choroidal neovascularization. Br J Pharmacol 179:5109–5131. https://doi.org/10.1111/bph.15925
Rohlenova K, Goveia J, García-Caballero M, Subramanian A, Kalucka J, Treps L, Falkenberg KD, de Rooij L, Zheng Y, Lin L, Sokol L, Teuwen LA, Geldhof V, Taverna F, Pircher A, Conradi LC, Khan S, Stegen S, Panovska D, De Smet F, Staal FJT, McLaughlin RJ, Vinckier S, Van Bergen T, Ectors N, De Haes P, Wang J, Bolund L, Schoonjans L, Karakach TK, Yang H, Carmeliet G, Liu Y, Thienpont B, Dewerchin M, Eelen G, Li X, Luo Y, Carmeliet P (2020) Single-cell RNA sequencing maps endothelial metabolic plasticity in pathological angiogenesis. Cell Metab 31:862-877.e14. https://doi.org/10.1016/j.cmet.2020.03.009
Wang Y, Zhang Q, Yang G, Wei Y, Li M, Du E, Li H, Song Z, Tao Y (2021) RPE-derived exosomes rescue the photoreceptors during retina degeneration: an intraocular approach to deliver exosomes into the subretinal space. Drug Deliv 28:218–228. https://doi.org/10.1080/10717544.2020.1870584
Xu W, Wu Y, Hu Z, Sun L, Dou G, Zhang Z, Wang H, Guo C, Wang Y (2019) Exosomes from microglia attenuate photoreceptor injury and neovascularization in an animal model of retinopathy of prematurity. Mol Ther Nucleic Acids 16:778–790. https://doi.org/10.1016/j.omtn.2019.04.029
Li D, Zhang J, Liu Z, Gong Y, Zheng Z (2021) Human umbilical cord mesenchymal stem cell-derived exosomal miR-27b attenuates subretinal fibrosis via suppressing epithelial-mesenchymal transition by targeting HOXC6. Stem Cell Res Ther 12:24. https://doi.org/10.1186/s13287-020-02064-0
Yu B, Shao H, Su C, Jiang Y, Chen X, Bai L, Zhang Y, Li Q, Zhang X, Li X (2016) Exosomes derived from MSCs ameliorate retinal laser injury partially by inhibition of MCP-1. Sci Rep 6:34562. https://doi.org/10.1038/srep34562
Cun Y, Jin Y, Wu D, Zhou L, Zhang C, Zhang S, Yang X, Zuhong W, Zhang P (2022) Exosome in crosstalk between inflammation and angiogenesis: a potential therapeutic strategy for stroke. Mediators Inflamm 2022:7006281. https://doi.org/10.1155/2022/7006281
Ansari MA, Thiruvengadam M, Venkidasamy B, Alomary MN, Salawi A, Chung IM, Shariati MA, Rebezov M (2022) Exosome-based nanomedicine for cancer treatment by targeting inflammatory pathways: current status and future perspectives. Semin Cancer Biol 86:678–696. https://doi.org/10.1016/j.semcancer.2022.04.005
Li Q, Pang L, Yang W, Liu X, Su G, Dong Y (2018) Long non-coding RNA of myocardial infarction associated transcript (LncRNA-MIAT) promotes diabetic retinopathy by upregulating transforming growth factor-β1 (TGF-β1) signaling. Med Sci Monit 24:9497–9503. https://doi.org/10.12659/msm.911787
Li EH, Huang QZ, Li GC, Xiang ZY, Zhang X (2017) Effects of miRNA-200b on the development of diabetic retinopathy by targeting VEGFA gene. Biosci Rep. https://doi.org/10.1042/bsr20160572
Pardue MT, Allen RS (2018) Neuroprotective strategies for retinal disease. Prog Retin Eye Res 65:50–76. https://doi.org/10.1016/j.preteyeres.2018.02.002
Zhang W, Wang Y, Kong Y (2019) Exosomes derived from mesenchymal stem cells modulate miR-126 to ameliorate hyperglycemia-induced retinal inflammation via targeting HMGB1. Invest Ophthalmol Vis Sci 60:294–303. https://doi.org/10.1167/iovs.18-25617
Steinle JJ (2020) Role of HMGB1 signaling in the inflammatory process in diabetic retinopathy. Cell Signal 73:109687. https://doi.org/10.1016/j.cellsig.2020.109687
Moisseiev E, Anderson JD, Oltjen S, Goswami M, Zawadzki RJ, Nolta JA, Park SS (2017) Protective effect of intravitreal administration of exosomes derived from mesenchymal stem cells on retinal ischemia. Curr Eye Res 42:1358–1367. https://doi.org/10.1080/02713683.2017.1319491
Yan B, Gao L, Huang Y, Wang X, Lang X, Yan F, Meng B, Sun X, Li G, Wang Y (2020) Exosomes derived from BDNF-expressing 293T attenuate ischemic retinal injury in vitro and in vivo. Aging. https://doi.org/10.18632/aging.202245
Pan D, Chang X, Xu M, Zhang M, Zhang S, Wang Y, Luo X, Xu J, Yang X, Sun X (2019) UMSC-derived exosomes promote retinal ganglion cells survival in a rat model of optic nerve crush. J Chem Neuroanat 96:134–139. https://doi.org/10.1016/j.jchemneu.2019.01.006
Prete M, Dammacco R, Fatone MC, Racanelli V (2016) Autoimmune uveitis: clinical, pathogenetic, and therapeutic features. Clin Exp Med 16:125–136. https://doi.org/10.1007/s10238-015-0345-6
Servat JJ, Mears KA, Black EH, Huang JJ (2012) Biological agents for the treatment of uveitis. Expert Opin Biol Ther 12:311–328. https://doi.org/10.1517/14712598.2012.658366
Bai L, Shao H, Wang H, Zhang Z, Su C, Dong L, Yu B, Chen X, Li X, Zhang X (2017) Effects of mesenchymal stem cell-derived exosomes on experimental autoimmune uveitis. Sci Rep 7:4323. https://doi.org/10.1038/s41598-017-04559-y
Kang M, Choi JK, Jittayasothorn Y, Egwuagu CE (2020) Interleukin 35-producing exosomes suppress neuroinflammation and autoimmune uveitis. Front Immunol 11:1051. https://doi.org/10.3389/fimmu.2020.01051
Jiang G, Yun J, Kaplan HJ, Zhao Y, Sun D, Shao H (2021) Vaccination with circulating exosomes in autoimmune uveitis prevents recurrent intraocular inflammation. Clin Exp Ophthalmol 49:1069–1077. https://doi.org/10.1111/ceo.13990
Jiang L, Cao H, Deng T, Yang M, Meng T, Yang H, Luo X (2021) Serum exosomal miR-377-3p inhibits retinal pigment epithelium proliferation and offers a biomarker for diabetic macular edema. J Int Med Res 49:3000605211002975. https://doi.org/10.1177/03000605211002975
Riau AK, Ong HS, Yam GHF, Mehta JS (2019) Sustained delivery system for stem cell-derived exosomes. Front Pharmacol 10:1368. https://doi.org/10.3389/fphar.2019.01368
Chaput N, Théry C (2011) Exosomes: immune properties and potential clinical implementations. Semin Immunopathol 33:419–440. https://doi.org/10.1007/s00281-010-0233-9
Deng CL, Hu CB, Ling ST, Zhao N, Bao LH, Zhou F, Xiong YC, Chen T, Sui BD, Yu XR, Hu CH (2021) Photoreceptor protection by mesenchymal stem cell transplantation identifies exosomal MiR-21 as a therapeutic for retinal degeneration. Cell Death Differ 28:1041–1061. https://doi.org/10.1038/s41418-020-00636-4
Pakravan K, Babashah S, Sadeghizadeh M, Mowla SJ, Mossahebi-Mohammadi M, Ataei F, Dana N, Javan M (2017) MicroRNA-100 shuttled by mesenchymal stem cell-derived exosomes suppresses in vitro angiogenesis through modulating the mTOR/HIF-1α/VEGF signaling axis in breast cancer cells. Cell Oncol (Dordr) 40:457–470. https://doi.org/10.1007/s13402-017-0335-7
Rajool Dezfuly A, Safaee A, Salehi H (2021) Therapeutic effects of mesenchymal stem cells-derived extracellular vesicles’ miRNAs on retinal regeneration: a review. Stem Cell Res Ther 12:530. https://doi.org/10.1186/s13287-021-02588-z
Dong X, Lei Y, Yu Z, Wang T, Liu Y, Han G, Zhang X, Li Y, Song Y, Xu H, Du M, Yin H, Wang X, Yan H (2021) Exosome-mediated delivery of an anti-angiogenic peptide inhibits pathological retinal angiogenesis. Theranostics 11:5107–5126. https://doi.org/10.7150/thno.54755
Aboul Naga SH, Dithmer M, Chitadze G, Kabelitz D, Lucius R, Roider J, Klettner A (2015) Intracellular pathways following uptake of bevacizumab in RPE cells. Exp Eye Res 131:29–41. https://doi.org/10.1016/j.exer.2014.12.010
Radhakrishnan K, Sonali N, Moreno M, Nirmal J, Fernandez AA, Venkatraman S, Agrawal R (2017) Protein delivery to the back of the eye: barriers, carriers and stability of anti-VEGF proteins. Drug Discov Today 22:416–423. https://doi.org/10.1016/j.drudis.2016.10.015
Sanghani A, Andriesei P, Kafetzis KN, Tagalakis AD, Yu-Wai-Man C (2022) Advances in exosome therapies in ophthalmology-from bench to clinical trial. Acta Ophthalmol 100:243–252. https://doi.org/10.1111/aos.14932
Hood JL (2016) Post isolation modification of exosomes for nanomedicine applications. Nanomedicine 11:1745–1756. https://doi.org/10.2217/nnm-2016-0102
Vallée A (2022) Curcumin and Wnt/β-catenin signaling in exudative age-related macular degeneration (review). Int J Mol Med. https://doi.org/10.3892/ijmm.2022.5135
Sun D, Zhuang X, Xiang X, Liu Y, Zhang S, Liu C, Barnes S, Grizzle W, Miller D, Zhang HG (2010) A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol Ther 18:1606–1614. https://doi.org/10.1038/mt.2010.105
Tian T, Zhang HX, He CP, Fan S, Zhu YL, Qi C, Huang NP, Xiao ZD, Lu ZH, Tannous BA, Gao J (2018) Surface functionalized exosomes as targeted drug delivery vehicles for cerebral ischemia therapy. Biomaterials 150:137–149. https://doi.org/10.1016/j.biomaterials.2017.10.012
Haney MJ, Klyachko NL, Zhao Y, Gupta R, Plotnikova EG, He Z, Patel T, Piroyan A, Sokolsky M, Kabanov AV, Batrakova EV (2015) Exosomes as drug delivery vehicles for Parkinson’s disease therapy. J Control Release 207:18–30. https://doi.org/10.1016/j.jconrel.2015.03.033
Kimiz-Gebologlu I, Oncel SS (2022) Exosomes: large-scale production, isolation, drug loading efficiency, and biodistribution and uptake. J Control Release 347:533–543. https://doi.org/10.1016/j.jconrel.2022.05.027
Acknowledgements
We thank our many colleagues who have contributed to our studies on this research.
Funding
This project was funded by National Natural Science Foundation of China (82074276), Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202002), Science and Technology Program of Tianjin (22ZYJDSS00100), Innovation Research Project of Chinese People's Armed Police Force (ZZKY20222419), and Program for Science and Technology of Logistics University of Chinese People’s Armed Police Force (WHJ202301).
Author information
Authors and Affiliations
Contributions
XP: Conceptualization, Design, Writing—original draft preparation & editing. TZ: Writing-editing. RL and XJ: Writing-review & editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
No animals was used for the research described in the article.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peng, X., Zhang, T., Liu, R. et al. Potential in exosome-based targeted nano-drugs and delivery vehicles for posterior ocular disease treatment: from barriers to therapeutic application. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04798-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04798-w