Abstract
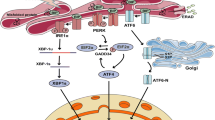
Septic cardiomyopathy (SCM) is one of the most serious complications of sepsis. The present study investigated the role and mechanism of upstream stimulatory factor 2 (USF2) in SCM. Serum samples were extracted from SCM patients and healthy individuals. A murine model of sepsis was induced by caecal ligation and puncture (CLP) surgery. Myocardial injury was examined by echocardiography and HE staining. ELISA assay evaluated myocardial markers (CK-MB, cTnI) and inflammatory cytokines (TNF-α, IL-1β, IL-18). Primary mouse cardiomyocytes were treated with lipopolysaccharide (LPS) to simulate sepsis in vitro. RT-qPCR and Western blot were used for analyzing gene and protein levels. CCK-8 assay assessed cell viability. NLRP3 was detected by immunofluorescence. ChIP, RIP and dual luciferase reporter assays were conducted to validate the molecular associations. USF2 was increased in serum from SCM patients, septic mice and primary cardiomyocytes. USF2 silencing improved the survival of septic mice and attenuated sepsis-induced myocardial pyroptosis and inflammation in vitro and in vivo. Mechanistically, USF2 could directly bind to the promoter of miR-206 to transcriptionally inhibit its expression. Moreover, RhoB was confirmed as a target of miR-206 and could promote ROCK activation and NLRP3 inflammasome formation. Moreover, overexpression of RhoB remarkably reversed the protection against LPS-induced inflammation and pyroptosis mediated by USF2 deletion or miR-206 overexpression in cardiomyocytes. The above findings elucidated that USF2 knockdown exerted a cardioprotective effect on sepsis by decreasing pyroptosis and inflammation via miR-206/RhoB/ROCK pathway, suggesting that USF2 may be a novel drug target in SCM.








Similar content being viewed by others
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author.
Abbreviations
- SCM:
-
Septic cardiomyopathy
- USF2:
-
Upstream stimulatory factor 2
- CLP:
-
Caecal ligation and puncture
- LPS:
-
Lipopolysaccharide
- NLRP3:
-
NOD-like receptor protein 3
- ASC:
-
Apoptosis-associated speck-like protein
- miRNAs:
-
MicroRNAs
- LVEF:
-
Left ventricular ejection fraction
- LVFS:
-
Left ventricular fraction shortening
- CK-MB:
-
Creatine kinase isoenzyme MB
- cTnI:
-
C-troponin I
- ELISA:
-
Enzyme-linked immunosorbent assay
- H&E:
-
Hematoxylin–eosin staining
- RT‑qPCR:
-
Reverse transcription quantitative polymerase chain reaction
- LV:
-
Left ventricular
- IF:
-
Immunofluorescence staining
- ChIP:
-
Chromatin immunoprecipitation
- SDS:
-
Sodium dodecyl sulfate
- RIP:
-
RNA immunoprecipitation
- SD:
-
Standard deviation
References
Salomão R, Ferreira BL, Salomão MC, Santos SS, Azevedo LCP, Brunialti MKC (2019) Sepsis: evolving concepts and challenges. Braz J Med Biol Res 52:e8595. https://doi.org/10.1590/1414-431x20198595
Fleischmann-Struzek C, Mellhammar L, Rose N, Cassini A, Rudd KE, Schlattmann P, Allegranzi B, Reinhart K (2020) Incidence and mortality of hospital- and ICU-treated sepsis: results from an updated and expanded systematic review and meta-analysis. Intensive Care Med 46:1552–1562. https://doi.org/10.1007/s00134-020-06151-x
Thompson K, Venkatesh B, Finfer S (2019) Sepsis and septic shock: current approaches to management. Intern Med J 49:160–170. https://doi.org/10.1111/imj.14199
Beesley SJ, Weber G, Sarge T, Nikravan S, Grissom CK, Lanspa MJ, Shahul S, Brown SM (2018) Septic cardiomyopathy. Crit Care Med 46:625–634. https://doi.org/10.1097/ccm.0000000000002851
Ravikumar N, Sayed MA, Poonsuph CJ, Sehgal R, Shirke MM, Harky A (2021) Septic cardiomyopathy: from basics to management choices. Curr Probl Cardiol 46:100767. https://doi.org/10.1016/j.cpcardiol.2020.100767
He Y, Hara H, Núñez G (2016) Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci 41:1012–1021. https://doi.org/10.1016/j.tibs.2016.09.002
Coll RC, Schroder K, Pelegrín P (2022) NLRP3 and pyroptosis blockers for treating inflammatory diseases. Trends Pharmacol Sci 43:653–668. https://doi.org/10.1016/j.tips.2022.04.003
Li N, Zhou H, Wu H, Wu Q, Duan M, Deng W, Tang Q (2019) STING-IRF3 contributes to lipopolysaccharide-induced cardiac dysfunction, inflammation, apoptosis and pyroptosis by activating NLRP3. Redox Biol 24:101215. https://doi.org/10.1016/j.redox.2019.101215
Zhang W, Tao A, Lan T, Cepinskas G, Kao R, Martin CM, Rui T (2017) Carbon monoxide releasing molecule-3 improves myocardial function in mice with sepsis by inhibiting NLRP3 inflammasome activation in cardiac fibroblasts. Basic Res Cardiol 112:16. https://doi.org/10.1007/s00395-017-0603-8
Feng D, Guo L, Liu J, Song Y, Ma X, Hu H, Liu J, Hao E (2021) DDX3X deficiency alleviates LPS-induced H9c2 cardiomyocytes pyroptosis by suppressing activation of NLRP3 inflammasome. Exp Ther Med 22:1389. https://doi.org/10.3892/etm.2021.10825
Liu F, Wang X, Zheng B, Li D, Chen C, Lee IS, Zhong J, Li D, Liu Y (2020) USF2 enhances the osteogenic differentiation of PDLCs by promoting ATF4 transcriptional activities. J Periodontal Res 55:68–76. https://doi.org/10.1111/jre.12689
Tan Y, Chen Y, Du M, Peng Z, Xie P (2019) USF2 inhibits the transcriptional activity of Smurf1 and Smurf2 to promote breast cancer tumorigenesis. Cell Signal 53:49–58. https://doi.org/10.1016/j.cellsig.2018.09.013
Sun J, Ge X, Wang Y, Niu L, Tang L, Pan S (2022) USF2 knockdown downregulates THBS1 to inhibit the TGF-β signaling pathway and reduce pyroptosis in sepsis-induced acute kidney injury. Pharmacol Res 176:105962. https://doi.org/10.1016/j.phrs.2021.105962
Zhang M, Yin C, Chen Y, Wang J, Jiang J (2022) Upstream stimulatory factor 2 (USF2) induced upregulation of triggering receptor expressed on myeloid cells 1 (TREM1) promotes endometritis by regulating toll-like receptor (TLR) 2/4-nuclear factor-kappaB (NF-κB) signaling pathway. Bioengineered 13:3609–3619. https://doi.org/10.1080/21655979.2022.2030619
Saliminejad K, Khorram Khorshid HR, Soleymani Fard S, Ghaffari SH (2019) An overview of microRNAs: biology, functions, therapeutics, and analysis methods. J Cell Physiol 234:5451–5465. https://doi.org/10.1002/jcp.27486
Jiang K, Guo S, Zhang T, Yang Y, Zhao G, Shaukat A, Wu H, Deng G (2018) Downregulation of TLR4 by miR-181a provides negative feedback regulation to lipopolysaccharide-induced inflammation. Front Pharmacol 9:142. https://doi.org/10.3389/fphar.2018.00142
van der Heide V, Möhnle P, Rink J, Briegel J, Kreth S (2016) Down-regulation of MicroRNA-31 in CD4+ T cells contributes to immunosuppression in human sepsis by promoting TH2 skewing. Anesthesiology 124:908–922. https://doi.org/10.1097/aln.0000000000001031
Liu R, Zheng S, Peng S, Yu Y, Fang J, Tan S, Yao F, Guo Z, Shao Y (2020) Prognostic value and clinicopathological features of MicroRNA-206 in various cancers: a meta-analysis. Biomed Res Int 2020:2159704. https://doi.org/10.1155/2020/2159704
Liang G, Wu Y, Guan Y, Dong Y, Jiang L, Mao G, Wu R, Huang Z, Jiang H, Qi L, Tang J (2020) The correlations between the serum expression of miR-206 and the severity and prognosis of sepsis. Ann Palliat Med 9:3222–3234. https://doi.org/10.21037/apm-20-1391
Zhou J, Fu Y, Liu K, Hou L, Zhang W (2019) miR-206 regulates alveolar type II epithelial cell Cx43 expression in sepsis-induced acute lung injury. Exp Ther Med 18:296–304. https://doi.org/10.3892/etm.2019.7551
Zhang X, Huang Z, Wang Y, Wang T, Li J, Xi P (2021) Long non-coding RNA RMRP contributes to sepsis-induced acute kidney injury. Yonsei Med J 62:262–273. https://doi.org/10.3349/ymj.2021.62.3.262
Yan Y, Lu K, Ye T, Zhang Z (2019) MicroRNA-223 attenuates LPS-induced inflammation in an acute lung injury model via the NLRP3 inflammasome and TLR4/NF-κB signaling pathway via RHOB. Int J Mol Med 43:1467–1477. https://doi.org/10.3892/ijmm.2019.4075
Zhang N, Fu L, Bu Y, Yao Y, Wang Y (2017) Downregulated expression of miR-223 promotes Toll-like receptor-activated inflammatory responses in macrophages by targeting RhoB. Mol Immunol 91:42–48. https://doi.org/10.1016/j.molimm.2017.08.026
Wu B, Song H, Fan M, You F, Zhang L, Luo J, Li J, Wang L, Li C, Yuan M (2020) Luteolin attenuates sepsis-induced myocardial injury by enhancing autophagy in mice. Int J Mol Med 45:1477–1487. https://doi.org/10.3892/ijmm.2020.4536
Song G, Wang L (2009) Nuclear receptor SHP activates miR-206 expression via a cascade dual inhibitory mechanism. PLoS ONE 4:e6880. https://doi.org/10.1371/journal.pone.0006880
Ackerman MH, Ahrens T, Kelly J, Pontillo A (2021) Sepsis. Crit Care Nurs Clin North Am 33:407–418. https://doi.org/10.1016/j.cnc.2021.08.003
L’Heureux M, Sternberg M, Brath L, Turlington J, Kashiouris MG (2020) Sepsis-induced cardiomyopathy: a comprehensive review. Curr Cardiol Rep 22:35. https://doi.org/10.1007/s11886-020-01277-2
Hollenberg SM, Singer M (2021) Pathophysiology of sepsis-induced cardiomyopathy. Nat Rev Cardiol 18:424–434. https://doi.org/10.1038/s41569-020-00492-2
Huang Y, Xu W, Zhou R (2021) NLRP3 inflammasome activation and cell death. Cell Mol Immunol 18:2114–2127. https://doi.org/10.1038/s41423-021-00740-6
Dai S, Ye B, Chen L, Hong G, Zhao G, Lu Z (2021) Emodin alleviates LPS-induced myocardial injury through inhibition of NLRP3 inflammasome activation. Phytother Res 35:5203–5213. https://doi.org/10.1002/ptr.7191
Liu JJ, Li Y, Yang MS, Chen R, Cen CQ (2020) SP1-induced ZFAS1 aggravates sepsis-induced cardiac dysfunction via miR-590–3p/NLRP3-mediated autophagy and pyroptosis. Arch Biochem Biophys 695:108611. https://doi.org/10.1016/j.abb.2020.108611
Qi L, Higgins CE, Higgins SP, Law BK, Simone TM, Higgins PJ (2014) The basic helix-loop-helix/leucine zipper transcription factor USF2 integrates serum-induced PAI-1 expression and keratinocyte growth. J Cell Biochem 115:1840–1847. https://doi.org/10.1002/jcb.24861
Hu D, Tjon EC, Andersson KM, Molica GM, Pham MC, Healy B, Murugaiyan G, Pochet N, Kuchroo VK, Bokarewa MI, Weiner HL (2020) Aberrant expression of USF2 in refractory rheumatoid arthritis and its regulation of proinflammatory cytokines in Th17 cells. Proc Natl Acad Sci U S A 117:30639–30648. https://doi.org/10.1073/pnas.2007935117
Zeng HB, Dong LQ, Huang YL, Xu C, Zhao XH, Wu LG (2021) USF2 reduces BMP3 expression via transcriptional activation of miR-34a, thus promoting osteogenic differentiation of BMSCs. J Bone Miner Metab 39:997–1008. https://doi.org/10.1007/s00774-021-01254-x
Zhang D, Wang Q, Qiu X, Chen Y, Yang X, Guan Y (2022) Remifentanil protects heart from myocardial ischaemia/reperfusion (I/R) injury via miR-206-3p/TLR4/NF-κB signalling axis. J Pharm Pharmacol 74:282–291. https://doi.org/10.1093/jpp/rgab151
Jing H, Wang C, Zhao L, Cheng J, Qin P, Lin H (2021) Propofol protects cardiomyocytes from hypoxia/reoxygenation injury via regulating MALAT1/miR-206/ATG3 axis. J Biochem Mol Toxicol 35:e22880. https://doi.org/10.1002/jbt.22880
Li Q, Zhu L, Niu F, Li Q, Wang C, Yang H, Gao C (2021) Histone deacetylase HDAC4 participates in the pathological process of myocardial ischemia-reperfusion injury via MEKK1/JNK pathway by binding to miR-206. Cell Death Discov 7:240. https://doi.org/10.1038/s41420-021-00601-1
Wang X, Bai M (2021) CircTM7SF3 contributes to oxidized low-density lipoprotein-induced apoptosis, inflammation and oxidative stress through targeting miR-206/ASPH axis in atherosclerosis cell model in vitro. BMC Cardiovasc Disord 21:51. https://doi.org/10.1186/s12872-020-01800-x
Hu X, Ma R, Fu W, Zhang C, Du X (2019) LncRNA UCA1 sponges miR-206 to exacerbate oxidative stress and apoptosis induced by ox-LDL in human macrophages. J Cell Physiol 234:14154–14160. https://doi.org/10.1002/jcp.28109
Zhou P, Xu P, Yu W, Li H (2022) MiR-206 improves intervertebral disk degeneration by targeting GJA1. J Orthop Surg Res 17:157. https://doi.org/10.1186/s13018-022-03044-1
Ueyama T (2019) Rho-Family Small GTPases: From Highly Polarized Sensory Neurons to Cancer Cells. Cells. https://doi.org/10.3390/cells8020092
Gutierrez E, Cahatol I, Bailey CAR, Lafargue A, Zhang N, Song Y, Tian H, Zhang Y, Chan R, Gu K, Zhang ACC, Tang J, Liu C, Connis N, Dennis P, Zhang C (2019) Regulation of RhoB Gene expression during tumorigenesis and aging process and its potential applications in these processes. Cancers (Basel). https://doi.org/10.3390/cancers11060818
Li Z, Huang B, Yi W, Wang F, Wei S, Yan H, Qin P, Zou D, Wei R, Chen N (2021) Identification of potential early diagnostic biomarkers of sepsis. J Inflamm Res 14:621–631. https://doi.org/10.2147/jir.S298604
Wang XH, Wang Y, Diao F, Lu J (2013) RhoB is involved in lipopolysaccharide-induced inflammation in mouse in vivo and in vitro. J Physiol Biochem 69:189–197. https://doi.org/10.1007/s13105-012-0201-z
Jianjun Z, Baochun Z, Limei M, Lijun L (2021) Exploring the beneficial role of ROCK inhibitors in sepsis-induced cerebral and cognitive injury in rats. Fundam Clin Pharmacol 35:882–891. https://doi.org/10.1111/fcp.12645
Cinel I, Ark M, Dellinger P, Karabacak T, Tamer L, Cinel L, Michael P, Hussein S, Parrillo JE, Kumar A, Kumar A (2012) Involvement of Rho kinase (ROCK) in sepsis-induced acute lung injury. J Thorac Dis 4:30–39. https://doi.org/10.3978/j.issn.2072-1439.2010.08.04
Zhang S, Zhu L, Dai H, Pan L (2021) Silencing ROCK1 ameliorates ventilator-induced lung injury in mice by inhibiting macrophages’ NLRP3 signaling. Int Immunopharmacol 101:108208. https://doi.org/10.1016/j.intimp.2021.108208
Acknowledgements
We would like to give our sincere gratitude to the reviewers for their constructive comments.
Funding
This work was supported by The National Natural Science Foundation of China (Grant No. 82060067), The Natural Science Foundation of Jiangxi Province (20212BAB216039), The project of Educational Commission of Jiangxi Province (GJJ190016) and The Project of Jiangxi Provincial Health Commission (20203096, 20203092).
Author information
Authors and Affiliations
Contributions
XP: conception and design of study. WD, JW, JC, WL, JY: acquisition of data. RL, XD, XF, TL, WW: analysis and interpretation of data. WD, RL: drafting the manuscript. XP: revising the manuscript critically for important intellectual content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of The first affiliated hospital of Nanchang University. Each patient provided written informed consent. All the animal procedures were performed in accordance with the Guiding Principles in the Use and Care of Animals and was authorized by the Animal Ethics Committee of the first affiliated hospital of Nanchang University.
Consent for publication
All participants were informed and gave written consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dong, W., Liao, R., Weng, J. et al. USF2 activates RhoB/ROCK pathway by transcriptional inhibition of miR-206 to promote pyroptosis in septic cardiomyocytes. Mol Cell Biochem (2023). https://doi.org/10.1007/s11010-023-04781-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11010-023-04781-5