Abstract
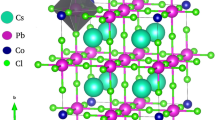
Semiconductor materials have a great potential to be used as a photocatalyst in several applications from dye degradation and water treatment up to solar cells. All-inorganic halide perovskite CsPbI3 and TiO2 with anatase structure were synthesized, studied, and compared as single samples and also forming heterostructures and composites. Structural, morphological, and optical characterizations reveal the successful synthesis of CsPbI3 and TiO2 compounds and the formation of both composites and heterostructures CsPbI3/TiO2. Methylene blue organic dye was used as a model for the study and evaluation of the photocatalytic activity exhibited by the produced semiconducting samples. The photocatalytic activity for MB degradation in methanol was investigated separately for TiO2 and CsPbI3 and their formation as composites and heterostructures. We have observed that when CsPbI3 perovskite is combined with TiO2, a cooperative mechanism involving the formation of intermediate phases promotes photobleaching with a kinetic constant rate much higher than both compounds separated or forming heterostructures. The CsPbI3/TiO2 causes MB mineralization by an oxygen-dependent mechanism. On the other hand, very high constant rate of the MB photodegradation can be observed by CsPbI3 perovskite even in a solution without the presence of dissolved oxygen. The presence of structural defects interstitials, vacancies, and under-coordinated Pb2+ ions on the surface of the perovskite particles may be formed during light irradiation and act as catalytic centers. The kinetic constant rate and the mechanism of MB photobleaching and the occurrence of dye mineralization can be tuned in feasible by simple strategies involving the formation of heterostructure and composites.
Graphical abstract










Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author (J.A.S.) upon reasonable request.
References
Shen S, Kronawitter C, Kiriakidis G (2017) An overview of photocatalytic materials. J Mater 3(1):1–2. https://doi.org/10.1016/j.jmat.2016.12.004
Fresno F, Portela R, Suárez S, Coronado JM (2014) Photocatalytic materials: recent achievements and near future trends. J Mater Chem A 2(9):2863–2884. https://doi.org/10.1039/c3ta13793g
Serpone N (2012) Emeline AV (2012) semiconductor photocatalysis: past, present, and future outlook. J Phys Chem Lett 3(5):673–677. https://doi.org/10.1021/jz300071j
Stranks SD, Snaith HJ (2015) Metal-halide perovskites for photovoltaic and light-emitting devices. Nat Nanotechnol 10:391–402. https://doi.org/10.1038/nnano.2015.90
Veiga ET, Fernandes SL, Graeff CFO, Polo AS (2021) Compact TiO2 blocking-layer prepared by LbL for perovskite solar cells. Sol Energy 214:510–516. https://doi.org/10.1016/j.solener.2020.11.024
Kamat PV (2007) Meeting the clean energy demand: nanostructure architectures for solar energy conversion. J Phys Chem C 111:2834–2860. https://doi.org/10.1021/jp066952u
Zhu D, Zhou Q (2019) Action and mechanism of semiconductor photocatalysis on degradation of organic pollutants in water treatment: a review. Environ Nanotechnol Monit Manag 100255:1–11. https://doi.org/10.1016/j.enmm.2019.100255
Guo Q, Zhou C, Ma Z, Ren Z, Fan H, Yang X (2016) Elementary photocatalytic chemistry on TiO2 surfaces. Chem Soc Rev 45(13):3701–3730. https://doi.org/10.1039/c5cs00448a
Panayotov DA, Burrows SP, Morris JR (2012) Photooxidation mechanism of methanol on rutile TiO2 nanoparticles. J Phys Chem C 116(11):6623–6635. https://doi.org/10.1021/jp209215c
Zhou H, Qu Y, Zeid T, Duan X (2012) Towards highly eficiente photocatalysts using semiconductor nanoarchitectures. Energy Environ Sci 5:6732–6743. https://doi.org/10.1039/C2EE03447F
Huang H, Pradhan B, Hofkens J, Roeffaers MBJ, Steele JA (2020) Solar-driven metal halide perovskite photocatalysis: design, stability, and performance. ACS Energy Lett 5:1107–1123. https://doi.org/10.1021/acsenergylett.0c00058
Manos D, Miserli K, Konstantinou I (2020) Perovskite and spinel catalysts for sulfate radical-based advanced oxidation of organic pollutants in water and wastewater systems. Catalysts 10(11):1299. https://doi.org/10.3390/catal10111299
Askeland DR (2003) The science and engineering of materials, 3rd edn. Springer, Dordrecht, pp 1–4
Sze SM, Kwok K (2006) Physics of semiconductor devices 2006, 1st edn. Wiley, New York
Ha ST, Su R, Xing J, Zhang Q, Xiong Q (2017) Metal halide perovskite nanomaterials: synthesis and applications. Chem Sci 8:2522–2536. https://doi.org/10.1039/c6sc04474c
Brenner TM, Egger DA, Kronik L, Hodes G, Cahen D (2016) Hybrid organic-inorganic perovskites: low-cost semiconductors with intriguing charge-transport properties. Nat Rev Mater 1:1–17. https://doi.org/10.1038/natrevmats.2015.7
Herz LM (2016) Charge-carrier dynamics in organic-inorganic metal halide perovskites. Annu Rev Phys Chem 67:65–89. https://doi.org/10.1146/annurev-physchem-040215-112222
Wehrenfennig C, Eperon GE, Johnston MB, Snaith HJ, Herz LM (2014) High charge carrier mobilities and lifetimes in organolead trihalide perovskites. Adv Mater 26:1584–1589. https://doi.org/10.1002/adma.201305172
Jin H, Debroye E, Keshavarz M, Scheblykin IG, Roeffaers MBJ, Hofkens J (2020) Steele JA (2002) It’s a trap! On the nature of localised states and charge trapping in lead halide perovskites. Mater Horiz 7:397–410. https://doi.org/10.1039/c9mh00500e
Ma T, Wang S, Zhang Y, Zhang K, Yi L (2019) The development of all-inorganic CsPbX3 perovskite solar cells. J Mater Sci. https://doi.org/10.1007/s10853-019-03974-y
Huynh KA, Nguyen DLT, Nguyen V, Vo DN, Trinh QT, Nguyen TP, Van Le Q (2020) Halide perovskite photocatalysis: progress and perspectives. J Chem Technol Biotechnol. https://doi.org/10.1002/jctb.6342
Gomez CMR, Pan S, Braga HM, Oliveira LS, Dalpian GM, Biesold-McGee GV, Lin Z, Santos SF, Souza JA (2020) A possible charge transfer induced conductivity enhancement in TiO2 microtubes decorated with perovskite CsPbBr3 nanocrystals. Langmuir 36:5408–5416. https://doi.org/10.1021/acs.langmuir.9b03871
Wada N, Yokomizo Y, Yogi C, Katayama M, Tanaka A, Kojima K, Ozutsumi K (2018) Effect of adding Au nanoparticles to TiO2 films on crystallization, phase transformation, and photocatalysis. J Mater Res 33(04):467–481. https://doi.org/10.1557/jmr.2018.16
Huang A, He Y, Zhou Y, Zhou Y, Yang Y, Zhang J, Yang J (2018) A review of recent applications of porous metals and metal oxide in energy storage, sensing and catalysis. J Mater Sci. https://doi.org/10.1007/s10853-018-2961-5
Nakata K, Fujishima A (2012) TiO2 photocatalysis: design and applications. J Photochem Photobiol, C 13(3):169–189. https://doi.org/10.1016/j.jphotochemrev.2012.06.001
Bakbolat B, Daulbayev C, Sultanov F, Beissenov R, Umirzakov A, Mereke A, Chuprakov I (2020) Recent developments of TiO2-based photocatalysis in the hydrogen evolution and photodegradation: a review. Nanomaterials 10(9):1790. https://doi.org/10.3390/nano10091790
Molina J, Zúñiga C, Moreno M, Calleja W, Rosales P, Ambrosio R, Sánchez JL (2014) Physical and electrical characterization of TiO2 particles after high temperature processing and before and after ultraviolet irradiation. Can J Phys 92(7/8):832–837. https://doi.org/10.1139/cjp-2013-0603
Fox MA, Dulay MT (1993) Heterogeneous photocatalysis. Chem Soc Rev 93:341–357. https://doi.org/10.1021/cr00017a016
Zhao Y, Wang C, Hu X, Fan J (2021) Recent progress in CsPbX3 perovskite nanocrystals for enhanced stability and photocatalytic applications. ChemNanoMat 7(7):789–804. https://doi.org/10.1002/cnma.202100094
Nawaz A, Kuila A, Mishra NS, Leong KH, Sim LC, Saravanan P, Jang M (2019) Challenges and implication of full solar spectrum-driven photocatalyst. Rev Chem Eng 37(4):533–560. https://doi.org/10.1515/revce-2018-0069
Qian R, Zong H, Schneider J, Zhou G, Zhao T, Li Y, Hong Pan J (2018) Charge carrier trapping, recombination and transfer during TiO2 photocatalysis: an overview. Catal Today 335:1–47. https://doi.org/10.1016/j.cattod.2018.10.053
Kronawitter CX, Vayssieres L, Shen SH, Guo LJ, Wheeler DA, Zhang JZ, Antoun BR, Mao SS (2011) A perspective on solar-driven water splitting with all-oxide hetero-nanostructures. Energy Environ Sci 4:3889–3999. https://doi.org/10.1039/c1ee02186a
Sombrio G, Pomar CAD, Oliveira LS, Freitas ALM, Souza FL, Souza JA (2019) Novel design of photocatalyst coaxial ferromagnetic core and semiconducting shell microwire architecture. J Catal 370:61–69. https://doi.org/10.1016/j.jcat.2018.12.010
Pelaez M, Nolan NT, Pillai SC (2012) A review on the visible light active titanium dioxide photocatalysts for environmental applications. Appl Catal B 125:331–349. https://doi.org/10.1016/j.apcatb.2012.05.036
Hirakawa T, Kominami H, Ohtani B, Nosaka Y (2001) Mechanism of photocatalytic production of active oxygens on highly crystalline TiO2 Particles by means of chemiluminescent probing and ESR spectroscopy. J Phys Chem B 105:6993–6999. https://doi.org/10.1021/jp0112929
Wang HL, Zhang LS, Chen ZG, Hu JQ, Li SJ, Wang ZH, Liu JS, Wang XC (2014) Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem Soc Rev 43:5234–5244. https://doi.org/10.1039/c4cs00126e
Callister WD, Rethwisch DG (2020) Materials science and engineering—an introduction, 10th edn. Wiley, Asia, p 713
Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW (2014) Understanding TiO2 photocatalysis: mechanisms and materials. Chem Rev 114(19):9919–9986. https://doi.org/10.1021/cr5001892
Zuo F, Wang L, Wu T, Zhang Z, Borchardt D, Feng P (2010) Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J Am Chem Soc 132:11856–11857. https://doi.org/10.1021/ja103843d
Zhang X, Zhang L (2010) Electronic and band structure tuning of ternary semiconductor photocatalysts by self doping: the case of BiOI. J Phys Chem C 114:18198–18206. https://doi.org/10.1021/jp105118m
Luo H, Dimitrov S, Daboczi M, Kim JS, Guo Q, Fang Y (2020) Nitrogen-doped carbon dots/TiO2 nanoparticle composites for photoelectrochemical water oxidation. ACS Appl Nano Mater 3:3371–3381. https://doi.org/10.1021/acsanm.9b02412
Zhang X, Wang Y, Liu B, Sang Y, Liu H (2017) Heterostructures construction on TiO2 nanobelts: a powerful tool for building high-performance photocatalysts. Appl Catal B 202:620–641. https://doi.org/10.1016/j.apcatb.2016.09.068
Wang H, Zhang L, Chen Z, Hu J, Li S, Wang Z, Wang X (2014) Semiconductor heterojunction photocatalysts: design, construction, and photocatalytic performances. Chem Soc Rev 43(15):5234–5244. https://doi.org/10.1039/c4cs00126e
Yuan YP, Ruan LW, Barber J, Joachim Loo SC, Xue C (2014) Hetero-nanostructured suspended photocatalysts for solar-to-fuel conversion. Energy Environ Sci 7(12):3934–3951. https://doi.org/10.1039/c4ee02914c
Miao Z, Wang G, Li L, Wang C, Zhang X (2019) Fabrication of black TiO2/TiO2 homojunction for enhanced photocatalytic degradation. J Mater Sci. https://doi.org/10.1007/s10853-019-03900-2
Moniz SJA, Shevlin SA, Martin DJ, Guo ZX, Tang J (2015) Visible-light driven heterojunction photocatalysts for water splitting: a critical review. Energy Environ Sci 8(3):731–759. https://doi.org/10.1039/c4ee03271c
Eaimsumang S, Prataksanon P, Pongstabodee S, Luengnaruemitchai A (2019) Effect of acid on the crystalline phase of TiO2 prepared by hydrothermal treatment and its application in the oxidative steam reforming of methanol. Res Chem Intermed 46:1235–1254. https://doi.org/10.1007/s11164-019-04031-8
Li Y, Wang S, Lei D, He YB, Li B, Kang F (2017) Acetic acid-induced preparation of anatase TiO2 mesocrystals at low temperature for enhanced Li-ion storage. J Mater Chem A 5(24):12236–12242. https://doi.org/10.1039/c7ta02361h
Carvalho F, Liandra-Salvador E, Bettanin F, Souza JS, Homem-de-Mello P, Polo AS (2014) Synthesis, characterization and photoelectrochemical performance of a tris-heteroleptic ruthenium(II) complex having 4,7-dimethyl-1,10-phenanthroline. Inorg Chim Acta 414:145–152. https://doi.org/10.1016/j.ica.2014.02.002
Schneider JT, Firak DS, Ribeiro RR, Peralta-Zamora PG (2020) Use of scavenger agents in heterogeneous photocatalysis: truths, half-truths, and misinterpretations. Phys Chem Chem Phys 22:15723–15733. https://doi.org/10.1039/d0cp02411b
Wardman P (1989) Reduction potentials of one-electron couples involving free radicals in aqueous solution. J Phys Chem 18:1637–1755. https://doi.org/10.1063/1.555843
Kawai T, Sakata T (1980) Photocatalytic hydrogen production from liquid methanol and water. J Chem Soc, Chem Commun 15:694–695. https://doi.org/10.1039/c39800000694
Jiang H, Liu Y, Zang S, Li J, Wang H (2015) Microwave-assisted hydrothermal synthesis of Nd, N, and P tri-doped TiO2 from TiCl4 hydrolysis and synergetic mechanism for enhanced photoactivity under simulated sunlight irradiation. Mater Sci Semicond Process 40:822–831. https://doi.org/10.1016/j.mssp.2015.07.069
Howard CJ, Sabine TM, Dickson F (1991) Structural and thermal parameters for rutile and anatase. Acta Crystallogr B Struct Sci 47(4):462–468. https://doi.org/10.1107/s010876819100335x
Wu S, Luo X, Long Y, Xu B (2019) Exploring the phase transformation mechanism of titanium dioxide by high temperature in situ method. IOP Conf Ser: Mater Scie Eng 493:012010. https://doi.org/10.1088/1757-899x/493/1/012010
Stoumpos CC, Malliakas CD, Kanatzidis MG (2013) Semiconducting Tin and lead iodide perovskites with organic cations: phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg Chem 52(15):9019–9038. https://doi.org/10.1021/ic401215x
Li X, Yu JG, Low JX, Fang YP, Xiao J, Chen XB (2015) Engineering heterogeneous semiconductors for solar water splitting. J Mater Chem A 3:2485–2534. https://doi.org/10.1039/c4ta04461d
Park H, Park Y, Kim W, Choi W (2013) Surface modification of TiO2 photocatalyst for environmental applications. J Photochem Photobiol, C 15:1–20. https://doi.org/10.1016/j.jphotochemrev.2012.10.001
Zhang J, Zhou P, Liu J, Yu J (2014) New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys Chem Chem Phys 16(38):20382–20386. https://doi.org/10.1039/c4cp02201g
Chen X, Liu L, Peter YY (2011) Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331:746–750. https://doi.org/10.1126/science.1200448
Sadoughi G, Starr DE, Handick E, Stranks SD, Gorgoi M, Wilks RG, Snaith HJ (2015) Observation and mediation of the presence of metallic lead in organic-inorganic perovskite films. ACS Appl Mater Interfaces 7(24):13440–13444. https://doi.org/10.1021/acsami.5b02237
Tardivo JP, Del Giglio A, Oliveira CS, Gabrielli DS, Junqueira HC, Tada DB, Baptista MS (2005) Methylene blue in photodynamic therapy: from basic mechanisms to clinical applications. Photodiagn Photodyn Ther 2(3):175–191. https://doi.org/10.1016/s1572-1000(05)00097-9
Chiu YH, Chang TFM, Chen CY, Sone M, Hsu YJ (2019) Mechanistic insights into photodegradation of organic dyes using heterostructure photocatalysts. Catalysts 9(5):430. https://doi.org/10.3390/catal9050430
Mohsen-Nia M, Amiri H, Jazi B (2010) Dielectric constants of water, methanol, ethanol, butanol and acetone: measurement and computational study. J Solut Chem 39(5):701–708. https://doi.org/10.1007/s10953-010-9538-5
Guo L, Okinaka N, Zhang L, Watanabe S (2021) Facile synthesis of ZnFe2O4/SnO2 composites for efficient photocatalytic degradation of methylene blue. Mater Chem Phys 262:124273. https://doi.org/10.1016/j.matchemphys.2021.124273
Ganharul GKQ, Tofanello A, Bonadio A, Freitas ALM, Escote MT, Polo AS, Souza JA (2021) Disclosing the hidden presence of Ti3+ ions in different TiO2 crystal structures synthesized at low temperature and photocatalytic evaluation by methylene blue photobleaching. J Mater Res 36:3353–3365. https://doi.org/10.1557/s43578-021-00342-y
Pomar CD, Souza AT, Sombrio G, Souza FL, Bonvent JJ, Souza JA (2018) Synthesis of SnS and ZnS hollow microarchitectures decorated with nanostructures and their photocatalytic behavior for dye degradation. ChemistrySelect 3(13):3774–37803. https://doi.org/10.1002/slct.201800383
Nosaka Y, Nosaka A (2016) Understanding hydroxyl radical (•OH) generation processes in photocatalysis. ACS Energy Lett 1(2):356–359. https://doi.org/10.1021/acsenergylett.6b00174
Zhang J, He J, Yang L, Gan Z (2020) Photoluminescent spectral broadening of lead halide perovskite nanocrystals investigated by emission wavelength dependent lifetime. Molecules 25(5):1151. https://doi.org/10.3390/molecules25051151
Zhang Q, Tai M, Zhou Y, Zhou Y, Wei Y, Tan C, Lin H (2019) Enhanced photocatalytic property of γ-CsPbI3 perovskite nanocrystals with WS2. ACS Sustain Chem Eng 8(2):1219–1229. https://doi.org/10.1021/acssuschemeng.9b06451
Ren Y, Chen J, Ji D, Sun Y, Li C (2019) Improve the quality of HC(NH2)2PbIxBr3–x through iodine vacancy filling for stable mixed perovskite solar cells. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123273
Boyd CC, Cheacharoen R, Leijtens T, McGehee MD (2018) Understanding degradation mechanisms and improving stability of perovskite photovoltaics. Chem Rev 119:3418–3451. https://doi.org/10.1021/acs.chemrev.8b00336
Acknowledgements
The research described herein was conducted during a PhD scholarship financed by UFABC. This work is supported by the Brazilian agency CNPq under Grants Nos. 307950/2017-4 and 404951/2016-3 and by the FAPESP under Grants Nos. 2017/02317-2, 2020/09563-1, 2018/15682-3, and 2019/23277-4. The authors are grateful to the Multiuser Central Facilities of UFABC for the experimental support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that no conflicts of interest or competing interests exist.
Human and animal rights
The authors declare that no experiments involving human tissue were carried out.
Additional information
Handling Editor: Joshua Tong.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ganharul, G.K.Q., Tofanello, A., Bonadio, A. et al. Outstanding cooperation of all-inorganic CsPbI3 perovskite with TiO2 forming composites and heterostructures for photodegradation. J Mater Sci 57, 17363–17379 (2022). https://doi.org/10.1007/s10853-022-07737-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-022-07737-0