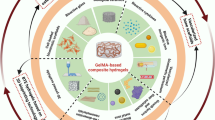
Abstract
Hydrogels can be designed as scaffolds in bone tissue engineering for more efficient healing of bone defects. Bone regeneration hydrogels have attracted extensive attention because of their good biocompatibility and excellent ability in promoting bone regeneration. This review will introduce the methods of bone regeneration hydrogels in the treatment of bone defects. Namely, promoting differentiation and proliferation of osteoblasts, promoting angiogenesis, regulating immune response and promoting mineralization. With the aim to deeply understand the development of bone regeneration hydrogels, we evaluate and summarize its characteristic, so as to help the future research.
Graphical Abstract















Similar content being viewed by others
References
Bartl R, Bartl C (2017) Structure and architecture of bone. In: Bone disorders. Springer, Cham. New York, pp 11–20. https://doi.org/10.1007/978-3-319-29182-6_2
Osman K, Gabr A, Haddad FS (2019) Bone healing. In: Paschos N, Bentley G (eds) General orthopaedics and basic science. Orthopaedic Study Guide Series. Springer, Cham. New York, pp 111–119. https://doi.org/10.1007/978-3-319-92193-8_14
Bartl R, Bartl C (2017) Fracture healing. In: Bone disorders. Springer, Cham. New York, pp 239–242. https://doi.org/10.1007/978-3-319-29182-6_35
Li JL, Melissa AK, David LS (2019) Fracture healing. In: Basic and applied bone biology, 2nd ed. Elsevier/Academic Press. London, pp 235–253. https://doi.org/10.1016/B978-0-12-813259-3.00012-9
Angshuman B, Ambalangodage C, Jayasuriya (2020) Recent trends in the application of widely used natural and synthetic polymer nanocomposites in bone tissue regeneration. Mater Sci Eng C 110:110698. https://doi.org/10.1016/j.msec.2020.110698
Zheng TY, Guo LY, Du ZY, Leng HJ, Cai Q, Yang XP (2021) Bioceramic fibrous scaffolds built with calcium silicate/hydroxyapatite nanofibers showing advantages for bone regeneration. CERAM INT 47:18920–18930. https://doi.org/10.1016/j.ceramint.2021.03.234
Li XF, Song T, Chen XN, Wang ML, Yang X, Xiao YM, Zhang XD (2019) Osteoinductivity of porous biphasic calcium phosphate ceramic spheres with nanocrystalline and their efficacy in guiding bone regeneration. ACS Appl Mater Interfaces 11:3722–3736. https://doi.org/10.1021/acsami.8b18525
He FP, Lu TL, Fang XB, Feng SH, Feng SL, Tian Y, Li YH, Zuo F, Deng X, Ye JD (2020) Novel extrusion-microdrilling approach to fabricate calcium phosphate-based bioceramic scaffolds enabling fast bone regeneration. ACS Appl Mater Interfaces 2020:32340–32351. https://doi.org/10.1021/acsami.0c07304
Aslankoohi N, Mequanint K (2020) Poly(ester amide)—bioactive glass hybrid biomaterials for bone regeneration and biomolecule delivery. ACS Appl Bio Mater 3:3621–3630. https://doi.org/10.1021/acsabm.0c00257
Zheng ZW, Chen YH, Guo B, Wang Y, Liu W, Sun J, Wang XS (2020) Magnesium-organic framework-based stimuli-responsive systems that optimize the bone microenvironment for enhanced bone regeneration. Chem Eng J 396:125241. https://doi.org/10.1016/j.cej.2020.125241
Seong YJ, Song EH, Park C, Lee H, Kang IG, Kim HE, Jeong SH (2020) Porous calcium phosphate—collagen composite microspheres for effective growth factor delivery and bone tissue regeneration. Mater Sci Eng C 109:110480. https://doi.org/10.1016/j.msec.2019.110480
Yasutaka M, Fumuki Y, Hideki N, Yuji M, Kousuke I, Toshihiko Y, Sakae T, Kazuhiko I, Yosuke O, Toru M, Taku S (2019) Multi-layered PLLA-nanosheets loaded with FGF-2 induce robust bone regeneration with controlled release in critical-sized mouse femoral defects. Acta Biomater 85:172–179. https://doi.org/10.1016/j.actbio.2018.12.031
Chen K, Lin XF, Zhang Q, Ni JF, Li JM, Xiao J, Wang Y, Ye YH, Chen L, Jin KK, Chen L (2015) Decellularized periosteum as a potential biologic scaffold for bone tissue engineering. Acta Biomater 19:46–55. https://doi.org/10.1016/j.actbio.2015.02.020
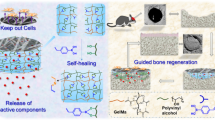
Bai X, Gao MZ, Syed S, Zhuang J, Xu XY, Zhang XQ (2018) Bioactive hydrogels for bone regeneration. Bioact Mater 3:401–417. https://doi.org/10.1016/j.bioactmat.2018.05.006
Saravanan S, Vimalraj S, Thanikaivelan P, Banudevi S, Manivasagam G (2019) A review on injectable chitosan/beta glycerophosphate hydrogels for bone tissue regeneration. Int J Biol Macromol 121:38–54. https://doi.org/10.1016/j.ijbiomac.2018.10.014
Marco CB, Vinoy T, Gudrun S, Yogesh KV, Chu TG, Michael JK, Gregg MJ (2012) Recent advances in the development of GTR/GBR membranes for periodontal regeneration—a materials perspective. Dent Mater 28:703–721. https://doi.org/10.1016/j.dental.2012.04.022
Afshar M, Dini G, Vaezifar S, Mehdikhani M, Movahedi B (2020) Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J Drug Deliv Sci Technol 56:101530. https://doi.org/10.1016/j.jddst.2020.101530
Zhai PS, Peng XX, Li BQ, Liu YP, Sun HC, Li XW (2020) The application of hyaluronic acid in bone regeneration. Int J Biol Macromol 151:1224–1239. https://doi.org/10.1016/j.ijbiomac.2019.10.169
Matthew RA, Janet MH, David BB (2004) Periosteum: biology, regulation, and response to osteoporosis therapies. Bone 35:1003–1012. https://doi.org/10.1016/j.bone.2004.07.014
Alonzo M, Primo FA, Kumar SA, Muldoff JA, Dominguez E, Fregoso G, Ortiz N, Weiss WM, Joddar B (2021) Bone tissue engineering techniques, advances, and scaffolds for treatment of bone defects. Curr opin Biomed Eng 17:100248. https://doi.org/10.1016/j.cobme.2020.100248
Hussain I, Sayed MS, Liu SL, Oderinde O, Yao F, Fu GD (2018) Glycogen-based self-healing hydrogels with ultra-stretchable, flexible, and enhanced mechanical properties via sacrificial bond interactions. Int J Biol Macromol 117:648–658. https://doi.org/10.1016/j.ijbiomac.2018.04.088
Sakai T, Matsunaga T, Yamamoto Y, Ito C, Yoshida R, Suzuki S, Sasaki N, Shibayama M, Chung U (2008) Design and fabrication of a high-strength hydrogel with ideally homogeneous network structure from tetrahedron-like macromonomers. Macromolecules 41:5379–5384. https://doi.org/10.1021/ma800476x
Gong JP, Katsuyama Y, Kurokawa T, Osada Y (2003) Double-network hydrogels with extremely high mechanical strength. Adv Mater 15:1155–1158. https://doi.org/10.1002/adma.200304907
Chen Q, Chen H, Zhu L, Zheng J (2015) Fundamentals of double network hydrogels. J Mat Chem B 3:3654–3676. https://doi.org/10.1039/C5TB00123D
Guo F, Huang KQ, Niu JJ, Kuang TR, Zheng YJ, Gu ZP, Zou J (2020) Enhanced osseointegration of double network hydrogels via calcium polyphosphate incorporation for bone regeneration. Int J Biol Macromol 151:1126–1132. https://doi.org/10.1016/j.ijbiomac.2019.10.155
Kaur G, Valarmathi MT, Potts JD, Jabbari E, Sabo-Attwood T, Qian W (2010) Regulation of osteogenic differentiation of rat bone marrow stromal cells on 2D nanorod substrates. Biomaterials 31:1732–1741. https://doi.org/10.1016/j.biomaterials.2009.11.041
Gaihre B, Liu XF, Li LL, Miller AL, Camilleri ET, Li Y, Waletzki B, Lu LC (2021) Bifunctional hydrogels for potential vascularized bone tissue regeneration. Mater Sci Eng C 124:112075. https://doi.org/10.1016/j.msec.2021.112075
Ou QM, Huang KQ, Fu CQ, Huang CL, Fang YF, Gu ZP, Wu J, Wang Y (2020) Nanosilver-incorporated halloysite nanotubes/gelatin methacrylate hybrid hydrogels with osteoimmunomodulatory and antibacterial activity for bone Regeneration. Chem Eng J 382:123019. https://doi.org/10.1016/j.cej.2019.123019
Huang KQ, Wu J, Gu ZP (2018) Black phosphorus hydrogels scaffolds enhance bone regeneration via a sustained supply of calcium-free phosphorus. ACS Appl Mater Interfaces 11:2908–2916. https://doi.org/10.1021/acsami.8b21179
Yi L, Zhong T, Huang YB, Huang SP (2020) Triiodothyronine promotes the osteoblast formation by activating autophagy. Biophys Chem 267:106483. https://doi.org/10.1016/j.bpc.2020.106483
Radhakrishnan J, Manigandan A, Chinnaswamy P, Subramanian A, Sethuraman S (2018) Gradient nano-engineered in situ forming composite hydrogels for osteochondral regeneration. Biomaterials 162:82–98. https://doi.org/10.1016/j.biomaterials.2018.01.056
Yaylaci SU, Sen M, Bulut O, Arslan E, Guler MO, Tekinay AB (2016) Chondrogenic differentiation of mesenchymal stem cells on glycosaminoglycan-mimetic peptide nanofibers. ACS Biomater Sci Eng 2:871–878. https://doi.org/10.1021/acsbiomaterials.6b00099
Schuurmans CCL, Mihajlovic M, Hiemstra C, Ito K, Hennink WE, Vermonden T (2021) Hyaluronic acid and chondroitin sulfate (meth)acrylate-based hydrogels for tissue engineering: synthesis, characteristics and pre-clinical evaluation. Biomaterials 268:120602. https://doi.org/10.1016/j.biomaterials.2020.120602
Liu X, Liu S, Yang R, Wang PH, Zhang WJ, Tan XY, Ren YH, Chi B (2021) Gradient chondroitin sulfate/poly (γ-glutamic acid) hydrogels inducing differentiation of stem cells for cartilage tissue engineering. Carbohydr Polym 270:118330. https://doi.org/10.1016/j.carbpol.2021.118330
Ma FB, Xia XY, Tang B (2019) Strontium chondroitin sulfate/silk fibroin blend membrane containing microporous structure modulates macrophage responses for guided bone regeneration. Mater Sci Eng C—Mater Biol Appl 117:111368. https://doi.org/10.1016/j.carbpol.2019.02.068
Zhang W, Zhao FJ, Huang DQ, Fu XL, Li X, Chen XF (2016) Strontium-substituted submicrometer bioactive glasses modulate macrophage responses for improved bone regeneration. ACS Appl Mater Interfaces 8:30747–30758. https://doi.org/10.1021/acsami.6b10378
Ma FB, Li SJ, Ruiz-Ortega LI, Zhang YJ, Xu L, Wang K, Lin LJ (2020) Effects of alginate/chondroitin sulfate-based hydrogels on bone defects healing. Mater Sci Eng C 116:111217. https://doi.org/10.1016/j.msec.2020.111217
Mierzwa AGH, Campos JF, Jesus MF, Nader HB, Lazaretti-Castro M (2017) Different doses of strontium ranelate and mechanical vibration modulate distinct responses in the articular cartilage of ovariectomized rats. Osteoarthr Cartil 25:1179–1188. https://doi.org/10.1016/j.joca.2017.02.793
Edward ON, Guleid A, Leila D, Obum U, Kevin WHL (2018) The roles of ions on bone regeneration. Drug Discov Today 23:879–890. https://doi.org/10.1016/j.drudis.2018.01.049
Xu L, Ma FB, Leung FKL, Qin CH, Lu WW, Tang B (2021) Chitosan-strontium chondroitin sulfate scaffolds for reconstruction of bone defects in aged rats. Carbohydr Polym 273:118532. https://doi.org/10.1016/j.carbpol.2021.118532
Lam J, Truong NF, Segura T (2014) Design of cell-matrix interactions in hyaluronic acid hydrogels scaffolds. Acta Biomater 10:1571–1580
An SW, Choi SJ, Min SJ, Cho SW (2021) Hyaluronic acid-based biomimetic hydrogels for tissue engineering and medical applications. Biotechnol Bioprocess Eng 26:503–516. https://doi.org/10.1007/s12257-020-0343-8
Abdul-Monem MM, Kamoun EA, Ahmed DM, El-Fakharany EM, Al-Abbassy FH, Aly HM (2021) Light-cured hyaluronic acid composite hydrogels using riboflavin as a photoinitiator for bone regeneration applications. J Taibah Univ Med Soc 16:529–539. https://doi.org/10.1016/j.jtumed.2020.12.021
Taz M, Makkar P, Imran KM, Jang DW, Kim YS, Lee BT (2019) Bone regeneration of multichannel biphasic calcium phosphate granules supplemented with hyaluronic acid. Mater Sci Eng C 99:1058–1066. https://doi.org/10.1016/j.msec.2019.02.051
Kaczmarek B, Sionkowska A, Kozlowska J, Osyczka A (2018) New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int J Biol Macromol 107:247–253. https://doi.org/10.1016/j.ijbiomac.2017.08.173
Lee SJ, Nah H, Heo DN, Kim KH, Seok JM, Heo M, Moon HJ, Lee D, Lee JS, An SY, Hwang YS, Ko WK, Kim SJ, Sohn S, Park SA, Park SY, Kwon IK (2020) Induction of osteogenic differentiation in a rat calvarial bone defect model using an in situ forming graphene oxide incorporated glycol chitosan/oxidized hyaluronic acid injectable hydrogels. Carbon 168:264–277. https://doi.org/10.1016/j.carbon.2020.05.022
Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A (2015) Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 73:254–271. https://doi.org/10.1016/j.biomaterials.2015.08.045
Nichol JW, Koshy ST, Bae H, Hwang CM, Yamanlar S, Khademhosseini A (2010) Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 31:5536–5544. https://doi.org/10.1016/j.biomaterials.2010.03.064
Neffe AT, Zaupa A, Pierce BJ, Hofmann D, Lendlein A (2010) Knowledge-based tailoring of gelatin-based materials by functionalization with tyrosine-derived groups. Macromol Rapid Commun 31:1534–1539. https://doi.org/10.1002/marc.201000274
Gan DL, Xu T, Xing WS, Wang MH, Fang J, Wang KF, Ge X, Chen CW, Ren FZ, Tan H, Lu X (2019) Mussel-inspired dopamine oligomer intercalated tough and resilient gelatin methacryloyl (GelMA) hydrogels for cartilage regeneration. J Mater Chem B 7:1716–1725. https://doi.org/10.1039/c8tb01664j
Paul A, Manoharan V, Krafft D, Assmann A, Uquillas JA, Shin SR, Hasan A, Hussain MA, Memic A, Gaharwar AK (2016) Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J Mater Chem B 4:3544–3554. https://doi.org/10.1039/C5TB02745D
Elomaa L, Keshi E, Sauer IM, Weinhart M (2020) Development of GelMA/PCL and dECM/PCL resins for 3D printing of acellular in vitro tissue scaffolds by stereolithography. Mater Sci Eng C 112:110958. https://doi.org/10.1016/j.msec.2020.110958
Cao YY, Cheng P, Sang SB, Xiang C, An Y, Wei XC, Yan YY, Li PC (2021) 3D printed PCL/GelMA biphasic scaffold boosts cartilage regeneration using co-culture of mesenchymal stem cells and chondrocytes: In vivo study. Mater Des 210:110065. https://doi.org/10.1016/j.matdes.2021.110065
Ribeiro JS, Bordini EAF, Ferreira JA, Mei L, Dubey N, Fenno JC, Piva E, Lund RG, Schwendeman A, Bottino MC (2020) Injectable MMP-responsive nanotube-modified gelatin hydrogels for dental infection ablation. ACS Appl Mater Interfaces 12:16006–16017. https://doi.org/10.1021/acsami.9b22964
Zargar V, Asghari M, Dashti A (2015) A review on chitin and chitosan polymers: structure, chemistry, solubility, derivatives, and applications. Chem BioEng Rev 2:204–226. https://doi.org/10.1002/cben.201400025
Chen YQ, Udduttula A, Xie XL, Zhou M, Sheng WB, Yu F, Weng J, Wang DL, Teng B, Manivasagam G, Zhang JV, Ren PG, Kang B, Zeng H (2021) A novel photocrosslinked phosphate functionalized Chitosan-Sr5(PO4)2SiO4 composite hydrogels and in vitro biomineralization, osteogenesis, angiogenesis for bone regeneration application. Compos Pt B-Eng 222:109057. https://doi.org/10.1016/j.compositesb.2021.109057
Purohit SD, Singh H, Bhaskar R, Yadav I, Bhushan S, Gupta MK, Kumar A, Mishra NC (2020) Fabrication of graphene oxide and nanohydroxyapatite reinforced gelatin-alginate nanocomposite scaffold for bone tissue regeneration. Front Mater 7:250. https://doi.org/10.3389/fmats.2020.00250
Bundela H, Bajpai AK (2008) Designing of hydroxyapatite-gelatin based porous matrix as bone substitute: correlation with biocompatibility aspects. Express Polym Lett 2:201–213. https://doi.org/10.3144/expresspolymlett.2008.25
Deng LZ, Liu Y, Yang LQ, Yi JZ, Deng FL, Zhang LM (2020) Injectable and bioactive methylcellulose hydrogels carrying bone mesenchymal stem cells as a filler for critical-size defects with enhanced bone regeneration. Coll and Surf B Biointerfaces 194:111159. https://doi.org/10.1016/j.colsurfb.2020.111159
Pan YS, Zhao Y, Kuang R, Liu H, Sun D, Mao TJ, Jiang KX, Yang XT, Watanabe N, Mayo KH, Lin Q, Li J (2020) Injectable hydrogels-loaded nano-hydroxyapatite that improves bone regeneration and alveolar ridge promotion. Mater Sci Eng C—Mater Biol Appl 116:111158. https://doi.org/10.1016/j.msec.2020.111158
Kim EJ, Choi JS, Kim JS, Choi YC, Cho YW (2016) Injectable and thermosensitive soluble extracellular matrix and methylcellulose hydrogels for stem cell delivery in skin wounds. Biomacromolecules 17:4–11. https://doi.org/10.1021/acs.biomac.5b01566
Dhivya S, Saravanan S, Sastry TP, Selvamurugan N (2015) Nanohydroxyapatite reinforced chitosan composite hydrogels for bone tissue repair in vitro and in vivo. J Nanobiotechnol 13:40. https://doi.org/10.1186/s12951-015-0099-z
Rocha JHG, Lemos AF, Kannan S, Agathopoulos S, Ferreira JMF (2005) Hydroxyapatite scaffolds hydrothermally grown from aragonitic cuttlefish bones. J Mater Chem 15:5007–5011. https://doi.org/10.1039/B510122K
Haberko K, Bućko MM, Brzezińska-Miecznik J, Haberko M, Mozgawa W, Panz T, Pyda A, Zarębski J (2006) Natural hydroxyl apatite its behaviour during heat treatment. J Eur Ceram Soc 26:537–542. https://doi.org/10.1016/j.jeurceramsoc.2005.07.033
Idris A, Ibiyeye TH, Abubakar MB, Haruna S, Hindatu Y, Mohammed NJ, Sulaiman M (2014) From garbage to biomaterials: an overview on egg shell based hydroxyapatite. J Mater 2014:1–6. https://doi.org/10.1155/2014/802467
Arjama M, Mehnath S, Rajan M, Jeyaraj M (2021) Injectable cuttlefish HAP and macromolecular fibroin protein hydrogels for natural bone mimicking matrix for enhancement of osteoinduction progression. React Funct Pol 160:104841. https://doi.org/10.1016/j.reactfunctpolym.2021.104841
Davoudi S, Chin CY, Cooke MJ, Tam RY, Shoichet MS, Gilbert PM (2018) Muscle stem cell intramuscular delivery within hyaluronan methylcellulose improves engraftment efficiency and dispersion. Biomaterials 173:34–46
Choi WI, Hwang Y, Sahu A, Min K, Sung D, Tae G, Chang JH (2018) An injectable and physical levan-based hydrogel as a dermal filler for soft tissue augmentation. Biomater Sci 6:2627–2638. https://doi.org/10.1039/c8bm00524a
Liu TT, Jin MQ, Zhang YZ, Weng WX, Wang TL, Yang HZ, Zhou L (2021) K+/Sr2+/Na+ triple-doped hydroxyapatites/GelMA composite hydrogels scaffold for the repair of bone defects. Ceram Int 47:30929–30937. https://doi.org/10.1016/j.ceramint.2021.07.277
Chandra VS, Baskar G, Suganthi RV, Elayaraja K, Joshy A, Beaula SW, Mythili R, Venkatraman G, Kalkura NS (2012) Blood compatibility of iron-doped nanosize hydroxyapatite and its drug release. ACS Appl Mater Interfaces 4:1200–1210. https://doi.org/10.1021/am300140q
Ullah I, Gloria A, Zhang WC, Ullah MW, Wu B, Li WC, Domingos M, Zhang XL (2020) Synthesis and characterization of sintered Sr/Fe-modified hydroxyapatite bioceramics for bone tissue engineering applications. ACS Biomater Sci Eng 6:375–388. https://doi.org/10.1021/acsbiomaterials.9b01666
Roohani-Esfahani S, Wong KY, Lu ZF, Chen YJ, Li JJ, Gronthos S, Menicanin D, Shi J, Dunstan C, Zreiqat H (2014) Fabrication of a novel triphasic and bioactive ceramic and evaluation of its in vitro and in vivo cytocompatibility and osteogenesis. J Mater Chem B 2:1866–1878. https://doi.org/10.1039/C3TB21504K
Kumar A, Kargozar S, Baino F, Han SS (2019) Additive manufacturing methods for producing hydroxyapatite and hydroxyapatite-based composite scaffolds: a review. Front Mater 6:313. https://doi.org/10.3389/fmats.2019.00313
Sarin N, Kurakula M, Singh KJ, Kumar A, Singh D, Arora S (2021) Strontium and selenium doped bioceramics incorporated polyacrylamide-carboxymethylcellulose hydrogels scaffolds: mimicking key features of bone regeneration. J Asian Ceram Soc 9:531–548. https://doi.org/10.1080/21870764.2021.1898168
Kumar A, Han SS (2021) Enhanced mechanical, biomineralization, and cellular response of nanocomposite hydrogels by bioactive glass and halloysite nanotubes for bone tissue regeneration. Mater Sci Eng C—Mater Biol Appl 128:112236. https://doi.org/10.1016/j.msec.2021.112236
Lin BC, Hu HX, Deng ZW, Pang LB, Jiang HZ, Wang DP, Li JS, Liu ZT, Wang H, Zeng XQ (2020) Novel bioactive glass cross-linked PVA hydrogels with enhanced chondrogenesis properties and application in mice chondrocytes for cartilage repair. J Non-Cryst Solids 529:119594. https://doi.org/10.1016/j.jnoncrysol.2019.119594
Kumar A, Rao KM, Han SS (2017) Synthesis of mechanically stiff and bioactive hybrid hydrogels for bone tissue engineering applications. Chem Eng J 317:119–131. https://doi.org/10.1016/j.cej.2017.02.065
Kumar A, Rao KM, Han SS (2018) Mechanically viscoelastic nanoreinforced hybrid hydrogels composed of polyacrylamide, sodium carboxymethylcellulose, graphene oxide, and cellulose nanocrystals. Carbohydr Polym 193:228–238. https://doi.org/10.1016/j.carbpol.2018.04.004
Zhang LM, Xia JG, Zhao QH, Liu LW, Zhang ZJ (2010) Functional graphene oxide as a nanocarrier for controlled loading and targeted delivery of mixed anticancer drugs. Small 6:537–544. https://doi.org/10.1002/smll.200901680
Nayak TR, Anderson H, Makam VS, Khaw C, Bae S, Xu XF, Ee PLR, Ahn JH, Hong BH, Pastorin G, Özyilmaz B (2011) Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 5:4670–4678. https://doi.org/10.1021/nn200500h
Jiao DL, Zheng A, Liu Y, Zhang XK, Wang X, Wu JN, She WJ, Lv KG, Cao LY, Jiang XQ (2021) Bidirectional differentiation of BMSCs induced by a biomimetic procallus based on a gelatin-reduced graphene oxide reinforced hydrogel for rapid bone regeneration. Bioact Mater 6:2011–2028. https://doi.org/10.1016/j.bioactmat.2020.12.003
Neffe AT, Zaupa A, Pierce BF, Hoffman D, Lendlein A (2010) Knowledge-based tailoring of gelatin-based materials by functionalization with tyrosine-derived groups. Macromol Macromol Rapid Commun 31:1534–1539. https://doi.org/10.1002/marc.201000274
Purohit SD, Bhaskar R, Singh H, Yadav I, Gupta MK, Mishra NC (2019) Development of a nanocomposite scaffold of gelatin–alginate–graphene oxide for bone tissue engineering. Int J Biol Macromol 133:592–602. https://doi.org/10.1016/j.ijbiomac.2019.04.113
Jiang LB, Ding SL, Ding W, Su DH, Zhang FX, Zhang TW, Yin XF, Xiao L, Li YL, Yuan FL, Dong J (2021) Injectable Sericin Based Nanocomposite hydrogels for Multi-modal Imaging-guided Immunomodulatory Bone Regeneration. Chem Eng J 418:129323. https://doi.org/10.1016/j.cej.2021.129323
Lee WC, Lim CHYX, Shi H, Tang LAL, Wang Y, Lim CT, Loh KP (2011) Origin of enhanced 43 stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 5:7334–7341. https://doi.org/10.1021/nn202190c
Lambert H, Frassetto L, Moore JB, Torgerson D, Gannon R, Burckhardt P, Lanham-New S (2015) The effect of supplementation with alkaline potassium salts on bone metabolism: a meta-analysis. Osteoporos Int 26:1311–1318. https://doi.org/10.1007/s00198-014-3006-9
Bigi A, Boanini E, Gazzano M (2016) Ion substitution in biological and synthetic apatites. In: Biomineralization and biomaterials, Elsevier/Academic Press, pp 235–266
Wang YC, Newman MR, Benoit DSW (2018) Development of controlled drug delivery systems for bone fracture-targeted therapeutic delivery: a review. Eur J Pharm Biopharm 127:223–236. https://doi.org/10.1016/j.ejpb.2018.02.023
Liu S, Chen XY, Zhang Q, Wu W, Xin JY, Li JS (2014) Multifunctional hydrogels based on β-cyclodextrin with both biomineralization and anti-inflammatory properties. Carbohydr Polym 102:869–876. https://doi.org/10.1016/j.carbpol.2013.10.076
Hedges AR (1998) Industrial applications of cyclodextrins. Chem Rev 98:2035–2044. https://doi.org/10.1021/cr970014w
Huang L, Xin JY, Guo YC, Li JS (2010) A novel insulin oral delivery system assisted by cationic β-cyclodextrin polymers. J Appl Polym Sci 115:1371–1379. https://doi.org/10.1002/app.30775
Roth JA, Kim BG, Lin WL, Cho MI (1999) Melatonin promotes osteoblast differentiation and bone formation. J Biol Chem 274:22041–22047. https://doi.org/10.1074/jbc.274.31.22041
Terauchi M, Tamura A, Yamaguchi S, Yui N (2018) Enhanced cellular uptake and osteogenic differentiation efficiency of melatonin by inclusion complexation with 2-hydroxypropyl β-cyclodextrin. Int J Pharm 547:53–60. https://doi.org/10.1016/j.ijpharm.2018.05.063
Zhang JX, Ma PX (2013) Cyclodextrin-based supramolecular systems for drug delivery: recent progress and future perspective. Adv Drug Deliv Rev 65:1215–1233. https://doi.org/10.1016/j.addr.2013.05.001
Rosario CD, Rodríguez-Évora M, Reyes R, Simões S, Concheiro A, Évora C, Alvarez-Lorenzo C, Delgado A (2015) Bone critical defect repair with poloxamine–cyclodextrin supramolecular gels. Int J Pharm 495:463–473. https://doi.org/10.1016/j.ijpharm.2015.09.003
Liao J, Wu S, Li K, Fan YB, Dunne N, Li XM (2019) Peptide-modified bone repair materials: Factors influencing osteogenic activity. J Biomed Mater Res Part A 107:1491–1512. https://doi.org/10.1002/jbm.a.36663
Erak M, Bellmann-Sickert K, Els-Heindl S, Beck-Sickinger AG (2018) Peptide chemistry toolbox—transforming natural peptides into peptide therapeutics. Bioorg Med Chem 26:2759–2765. https://doi.org/10.1016/j.bmc.2018.01.012
Jabbari E (2013) Osteogenic peptides in bone regeneration. Curr Pharm Des 19:3391–3402. https://doi.org/10.2174/1381612811319190006
Saito A, Suzuki Y, Ogata SI, Ohtsuki C, Tanihana M (2005) Accelerated bone repair with the use of a synthetic BMP-2-derived peptide and bone-marrow stromal cells. J Biomed Mater Res Part A 72A:77–82. https://doi.org/10.1002/jbm.a.30208
Li RX, Sun Y, Cai ZW, Li Y, Sun J, Bi W, Yang F, Zhou QR, Ye TJ, Yu YC (2021) Highly bioactive peptide-HA photo-crosslinking hydrogels for sustained promoting bone regeneration. Chem Eng J 415:129015. https://doi.org/10.1016/j.cej.2021.129015
Almubarak S, Nethercott H, Freeberg M, Beaudon C, Jha A, Jackson W, Marcucio R, Miclau T, Healy K, Bahney C (2016) Tissue engineering strategies for promoting vascularized bone regeneration. Bone 83:197–209. https://doi.org/10.1016/j.bone.2015.11.011
Yin J, Gong G, Sun C, Yin ZY, Zhu C, Wang B, Hu Q, Zhu YR, Liu XH (2018) Angiopoietin 2 promotes angiogenesis in tissue-engineered bone and improves repair of bone defects by inducing autophagy. Biomed Pharm 105:932–939. https://doi.org/10.1016/j.biopha.2018.06.078
Seebach C, Henrich D, Kähling C, Wilhelm K, Tami AE, Alini M, Marzi I (2010) Endothelial progenitor cells and mesenchymal stem cells seeded onto beta-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng Part A 16:1961–1970. https://doi.org/10.1089/ten.TEA.2009.0715
Wang T, Guo S, Zhang H, Chen YQ, Cai Y (2020) Injectable hydrogels delivering bone morphogenetic protein-2, vascular endothelial growth factor, and adipose-derived stem cells for vascularized bone tissue engineering. J Drug Deliv Sci Technol 57:101637. https://doi.org/10.1016/j.jddst.2020.101637
Apte RS, Chen DS, Ferrara N (2019) VEGF in signaling and disease: beyond discovery and development. Cell 176:1248–1264. https://doi.org/10.1016/j.cell.2019.01.021
Wang KK, Cheng WN, Ding ZZ, Xu G, Zheng X, Li MR, Lu GZ, Lu Q (2020) Injectable silk/hydroxyapatite nanocomposite hydrogels with vascularization capacity for bone regeneration. J Mater Sci Technol 63:172–181. https://doi.org/10.1016/j.jmst.2020.02.030
Tan JL, Zhang M, Hai ZJ, Wu CF, Lin J, Kuang W, Tang H, Huang YL, Chen XD, Liang GL (2019) Sustained release of two bioactive factors from supramolecular hydrogels promotes periodontal bone regeneration. ACS Nano 13:5616–5622. https://doi.org/10.1021/acsnano.9b00788
Li GJ, An JD, Han XW, Zhang XL, Wang WJ, Wang SK (2019) Hypermethylation of microRNA-149 activates SDF-1/CXCR4 to promote osteogenic differentiation of mesenchymal stem cells. J Cell Physiol 234:23485–23494. https://doi.org/10.1002/jcp.28917
Ran QC, Yu YL, Chen WZ, Shen XK, Mu CY, Yuan Z, Tao BL, Hu Y, Yang WH, Cai KY (2018) Deferoxamine loaded titania nanotubes substrates regulate osteogenic and angiogenic differentiation of MSCs via activation of HIF-1α signaling. Mater Sci Eng C 91:44–54. https://doi.org/10.1016/j.msec.2018.04.098
Li PY, Sakuma K, Tsuchiya S, Sun LH, Hayamizu Y (2019) Fibroin-like peptides self-assembling on two-dimensional materials as a molecular scaffold for potential biosensing. ACS Appl Mater Interfaces 11:20670–20677. https://doi.org/10.1021/acsami.9b04079
Kong LZ, Wu Z, Zhao HK, Cui HM, Shen J, Chang J, Li HY, He YH (2018) Bioactive injectable hydrogels containing desferrioxamine and bioglass for diabetic wound healing. ACS Appl Mater Interfaces 10:30103–30114. https://doi.org/10.1021/acsami.8b09191
Yoshizawa S, Brown A, Barchowsky A, Sfeir C (2014) Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater 10:2834–2842. https://doi.org/10.1016/j.actbio.2014.02.002
Zhang XT, Huang PZ, Jiang GW, Zhang MD, Yu F, Dong XP, Wang LP, Chen YH, Zhang WT, Qi Y, Li WQ, Zeng H (2021) A novel magnesium ion-incorporating dual-crosslinked hydrogels to improve bone scaffold-mediated osteogenesis and angiogenesis. Mater Sci Eng C 121:111868. https://doi.org/10.1016/j.msec.2021.111868
Tan J, Wang DH, Cao HL, Qiao YQ, Zhu HQ, Liu XY (2018) Effect of local alkaline micro-environment on the behaviors of bacteria and osteogenic cells. ACS Appl Mater Interfaces 10:42018–42029. https://doi.org/10.1021/acsami.8b15724
Wei PF, Jing W, Yuan ZY, Huang YQ, Guan BB, Zhang WX, Zhang X, Mao JP, Cai Q, Chen DF, Yang XP (2019) Vancomycin- and strontium-loaded microspheres with multifunctional activities against bacteria, angiogenesis, and in osteogenesis for enhancing infected bone regeneration. ACS Appl Mater Interfaces 11:30596–30609. https://doi.org/10.1021/acsami.9b10219
Qiu PC, Li MB, Chen K, Fang B, Chen PF, Tang ZB, Lin XF, Fan SW (2020) Periosteal matrix-derived hydrogels promotes bone repair through an early immune regulation coupled with enhanced angio- and osteogenesis. Biomaterials 227:119552. https://doi.org/10.1016/j.biomaterials.2019.119552
Wang TT, Luu TU, Chen A, Khine M, Liu WF (2016) Topographical modulation of macrophage phenotype by shrink-film multi-scale wrinkles. Biomater Sci 4:948–952. https://doi.org/10.1039/c6bm00224b
Liu WJ, Sun J, Sun Y, Xiang Y, Yan YF, Han ZH, Bi W, Yang F, Zhou QR, Wang L, Yu YC (2020) Multifunctional injectable protein-based hydrogels for bone regeneration. Chem Eng J 394:124875. https://doi.org/10.1016/j.cej.2020.124875
Zhang DH, Chen Q, Zhang WJ, Liu HJ, Liu RH (2020) Silk-inspired beta-peptide materials resist fouling and the foreign-body response. Angew Chem Int Ed Engl 59:9586–9593. https://doi.org/10.1002/anie.202000416
Jetten N, Verbruggen S, Gijbels MJ, Post MJ, Winther MPJD, Donners MMPC (2014) Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17:109–118
Wang M, Yu YM, Dai K, Ma ZY, Liu Y, Wang J, Liu CS (2016) Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater Sci 4:1574–1583. https://doi.org/10.1039/c6bm00290k
He JH, Chen GB, Liu MY, Xu ZL, Chen H, Yang L, Lv YG (2020) Scaffold strategies for modulating immune microenvironment during bone regeneration. Mater Sci Eng C Mater Biol Appl 108:110411. https://doi.org/10.1016/j.msec.2019.110411
Huang LP, Zhang JH, Hu JF, Zhao TB, Gu ZP (2020) Biomimetic gelatin methacrylate/nano fish bone hybrid hydrogels for bone regeneration via osteoimmunomodulation. ACS Biomater Sci Eng 6:3270–3274. https://doi.org/10.1021/acsbiomaterials.0c00443
Zimmermann EA, Ritchie RO (2015) Bone as a structural material. Adv Healthc Mater 4:1287–1304. https://doi.org/10.1002/adhm.201500070
Bonucci E (2012) Bone mineralization. Front Biosci 17:100–128. https://doi.org/10.2741/3918
Estroff LA, Addadi L, Weiner S, Hamilton AD (2004) An organic hydrogels as a matrix for the growth of calcite crystals. Org Biomolecular Chem 2:137–141. https://doi.org/10.1039/b309731e
Song J, Malathong V, Bertozzi CR (2004) Mineralization of synthetic polymer scaffolds: a bottom-up approach for the development of artificial bone. J Am Chem Soc 127:3366–3372. https://doi.org/10.1021/ja043776z
Yao SS, Xu YF, Zhou YY, Shao CY, Liu ZM, Jin B, Zhao RB, Cao H, Pan HH, Tang RK (2019) Calcium phosphate nanocluster-loaded injectable hydrogels for bone regeneration. ACS Appl Bio Mater 2:4408–4417. https://doi.org/10.1021/acsabm.9b00270
Olszta MJ, Cheng XG, Jee SS, Kumar R, Kim YY, Kaufman MJ, Douglas EP, Gower LB (2007) Bone structure and formation: a new perspective. Mater Sci Eng R Rep 58:77–116. https://doi.org/10.1016/j.mser.2007.05.001
Niu LN, Jiao K, Ryou HJ, Yiu CKY, Chen JH, Breschi L, Arola DD, Pashley DH, Tay FR (2012) Multiphase intrafibrillar mineralization of collagen. Angew Chem Int Ed Engl 52:5762–5766. https://doi.org/10.1002/anie.201210259
Yu XH, Zhang DW, Zheng XL, Tang CK (2019) Cholesterol transport system: an integrated cholesterol transport model involved in atherosclerosis. Prog Lipid Res 73:65–91. https://doi.org/10.1016/j.plipres.2018.12.002
Dong ZH, Yang Q, Mei MY, Liu L, Sun JX, Zhao L, Zhou CC (2018) Preparation and characterization of fluoride calcium silicate composites with multi-biofunction for clinical application in dentistry. Compos Part B Eng 143:243–249. https://doi.org/10.1016/j.compositesb.2018.02.009
Zhang XT, He YY, Huang PZ, Jiang GW, Zhang MD, Yu F, Zhang WT, Fu G, Wang Y, Li WQ, Zeng H (2020) A novel mineralized high strength hydrogels for enhancing cell adhesion and promoting skull bone regeneration in situ. Compos Part B Eng 197:108183. https://doi.org/10.1016/j.compositesb.2020.108183
Huang L, Zhu ZY, Wu DW, Gan WD, Zhu SS, Li WQ, Tian JH, Li LH, Zhou CR, Lu L (2019) Antibacterial poly (ethylene glycol) diacrylate/chitosan hydrogels enhance mechanical adhesiveness and promote skin regeneration. Carbohydr Polym 225:115110. https://doi.org/10.1016/j.carbpol.2019.115110
Penido MGMG, Alon US (2012) Phosphate homeostasis and its role in bone health. Pediatr Nephrol 27:2039–2048. https://doi.org/10.1007/s00467-012-2175-z
Zhang R, Zhou XY, Zhang D, Lou WK, Zhai F, Chang K (2015) Electronic and magneto-optical properties of monolayer phosphorene quantum dots. 2D Mater 2:045012. https://doi.org/10.1088/2053-1583/2/4/045012
Chen WS, Ouyang J, Liu H, Chen M, Zeng K, Sheng JP, Liu ZJ, Han YJ, Wang LQ, Li J, Deng L, Liu YN, Guo SJ (2017) Black phosphorus nanosheet‐based drug delivery system for synergistic photodynamic/photothermal/chemotherapy of cancer. Adv Mater 29:1603864.1–1603864.7. https://doi.org/10.1002/adma.201603864
Shao JD, Xie HH, Huang H, Li ZB, Sun ZB, Xu YH, Xiao QL, Yu XF, Zhao YT, Zhang H, Wang HY, Chu PK (2016) Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat Commun 7:12967. https://doi.org/10.1038/ncomms12967
Ziletti A, Carvalho A, Campbell DK, Coker DF, Neto AHC (2015) Oxygen defects in phosphorene. Phys Rev Lett 114:046801. https://doi.org/10.1103/PhysRevLett.114.046801
Rau LR, Huang WY, Liaw JW, Tsai SW (2016) Photothermal effects of laser-activated surface plasmonic gold nanoparticles on the apoptosis and osteogenesis of osteoblast-like cells. Int J Nanomed 11:3461–3473. https://doi.org/10.2147/IJN.S108152
Yanagi T, Kajiya H, Kawaguchi M, Kido H, Fukushima T (2015) Photothermal stress triggered by near infrared-irradiated carbon nanotubes promotes bone deposition in rat calvarial defects. J Biomater Appl 29:1109–1118. https://doi.org/10.1177/0885328214556913
Huang JJ, Liu G, Song CY, Saiz E, Tomsia AP (2012) Role of molecular chemistry of degradable phema hydrogels in three-dimensional biomimetic mineralization. Chem Mater 24:1331–1337. https://doi.org/10.1021/cm203764f
Masuyama A, Shindoh A, Ono D, Okahara M (1989) Preparation and surface active properties of terminal amide type of alcohol ethoxylates. J Am Oil Chem Soc 66:834–837. https://doi.org/10.1007/BF02653680
Cheng RY, Xin TW, Liu LL, Wang F, Ye TJ, Deng LF (2020) Cui WG (2020) A “three-in-one” injectable hydrogels platform with osteogenesis, angiogenesis and antibacterial for guiding bone regeneration. Appl Mater Today 20:100763. https://doi.org/10.1016/j.apmt.2020.100763
Ma YF, Lin M, Huang GY, Li YH, Wang SQ, Bai GQ, Lu TJ, Xu F (2018) Spatiotemporal mechanical microenvironment: a hydrogels-based platform for guiding stem cell fate. Adv Mater 30:1705911. https://doi.org/10.1002/adma.201705911
Tong ZR, Jin LL, Oliveira JM, Reis RL, Zhong Q, Mao ZW, Gao CY (2021) Adaptable hydrogels with reversible linkages for regenerative medicine: Dynamic mechanical microenvironment for cells. Bioact Mater 6:1375–1387. https://doi.org/10.1016/j.bioactmat.2020.10.029
Hollister SJ (2005) Porous scaffold design for tissue engineering. Nat Mater 4:518–524
Tayalia P, Mooney DJ (2010) Controlled, Growth factor delivery for tissue engineering. Adv Mater 21:3269–3285. https://doi.org/10.1002/adma.200900241
Acknowledgements
This work was supported by National Natural Science Foundation of China (NSFC No. 52063006) and the Science and Technology Foundation of Guizhou Province (Grant Number 2019-112-016).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling Editor: Maude Jimenez.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiong, Y., Xiong, Y. Applications of bone regeneration hydrogels in the treatment of bone defects: a review. J Mater Sci 57, 887–913 (2022). https://doi.org/10.1007/s10853-021-06675-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-021-06675-7