Abstract
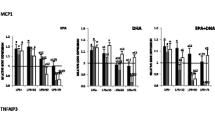
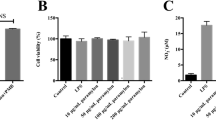
In recent years, there has been increasing interest in studying the anti-inflammatory activity of polyunsaturated fatty acid ethanolamides (N-acylethanolamines, NAE), which are highly active lipid mediators. The results of this study demonstrate that a dietary supplement (DS) of fatty acid-derived NAEs reduces LPS-induced inflammation. The processes of cell proliferation, as well as the dynamics of Iba-1-, CD68-, and CD163-positive macrophage activity within the thymus and spleen were studied. The production of pro-inflammatory cytokines (TNF, IL1β, IL6, and INFγ), ROS, NO, and nitrites was evaluated in the blood serum, thymus, and LPS-stimulated RAW264.7 mouse macrophages. In vitro and in vivo experiments have shown that DS (1) prevents LPS-induced changes in the morphological structure of the thymus and spleen; (2) levels out changes in cell proliferation; (3) inhibits the activity of Iba-1 and CD68-positive cells; (4) reduces the production of pro-inflammatory cytokines (TNF, IL1β, IL6, and INFγ), ROS, and CD68; and (5) enhances the activity of CD-163-positive cells. In general, the results of this study demonstrate the complex effect of DS on inflammatory processes in the central and peripheral immune systems.





Similar content being viewed by others
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
References
Austermann, J., J. Roth, and K. Barczyk-Kahlert. 2022. The good and the bad: Monocytes’ and macrophages’ diverse functions in inflammation. Cells 11: 1979. https://doi.org/10.3390/cells11121979. (PMID: 35741108).
Biswas, S.K., M. Chittezhath, I.N. Shalova, and J.Y. Lim. 2012. Macrophage polarization and plasticity in health and disease. Immunologic Research 53: 11–24. https://doi.org/10.1007/s12026-012-8291-9.
Blagov, A.V., A.M. Markin, A.I. Bogatyreva, T.V. Tolstik, V.N. Sukhorukov, and A.N. Orekhov. 2023. The role of macrophages in the pathogenesis of atherosclerosis. Cells 12 (4): 522. https://doi.org/10.3390/cells12040522.
Borges Da Silva, H., R. Fonseca, R.M. Pereira, A.A. Cassado, J.M. Álvarez, and M.R. D’Império Lima. 2015. Splenic macrophage subsets and their function during blood-borne infections. Frontiers in immunology 6: 480. https://doi.org/10.3389/fimmu.2015.00480. (PMID: 26441984).
Brown, I., M.G. Cascio, K.W.J. Wahle, R. Smoum, R. Mechoulam, R.A. Ross, R.G Pertwee and S.D. Heys. 2010. Cannabinoid receptor-dependent and -independent anti-proliferative effects of omega-3 ethanolamides in androgen receptor-positive and -negative prostate cancer cell lines. Carcinogenesis 31(9): 1584–1591. https://doi.org/10.1093/carcin/bgq151.
Calder, P.C. 2013. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? British Journal of Clinical Pharmacology 75: 645–662. https://doi.org/10.1111/j.1365-2125.2012.04374.x. (PMID: 22765297).
Calvo, M.J., M.S. Martinez, W. Torres, M. Chavez-Castillo, E. Luzardo, N. Villasmil, J. Salazar, M. Velasco, and V. Bermudez. 2017. Omega-3 polyunsaturated fatty acids and cardiovascular health: a molecular view into structure and function. Vessel Plus 1: 116- 128. https://doi.org/10.20517/2574-1209.2017.14.
Cassetta, L., E. Cassol, and G. Poli. 2011. Macrophage polarization in health and disease. The Scientific World Journal 11: 2391–2402. https://doi.org/10.1100/2011/213962. (PMID: 22194670).
Chávez-Galán, L., M.L. Olleros, D. Vesin, and I. Garcia. 2015. Much more than M1 and M2 macrophages, there are also CD169+ and TCR+ macrophages. Frontiers in Immunology 6: 263. https://doi.org/10.3389/fimmu.2015.00263. (PMID: 26074923).
Chen, L., H. Deng, H. Cui, J. Fang, Z. Zuo, J. Deng, Y. Li, X. Wang, and L. Zhao. 2018. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 9: 7204–7218. https://doi.org/10.18632/oncotarget.23208. (PMID: 29467962).
Chen, S., A.F. Saeed, Q. Liu, Q. Jiang, H. Xu, G. Guishan Xiao, and L. Rao. 2023. Macrophages in immunoregulation and therapeutics. Signal Transduction and Targeted Therapy 8: 207. https://doi.org/10.1038/s41392-023-01452-1. (PMID: 37211559).
Cutolo, M., R. Campitiello, E. Gotelli, and S. Soldano. 2022. The role of M1/M2 macrophage polarization in rheumatoid arthritis synovitis. Frontiers in Immunology 13: 867260. https://doi.org/10.3389/fimmu.2022.867260. (PMID: 35663975).
Dzhalilova, D.S., A.M. Kosyreva, M.E. Diatroptov, N.A. Zolotova, I.S. Tsvetkov, V.A. Mkhitarov, O.V. Makarova, and D.N. Khochanskiy. 2019. Morphological characteristics of the thymus and spleen and the subpopulation composition of lymphocytes in peripheral blood during systemic inflammatory response in male rats with different resistance to hypoxia. International Journal of Inflammation 2019, 7584685. https://doi.org/10.1155/2019/7584685. (PMID: 31057785).
Deng, Z. and S. Liy. 2021. Inflammation-responsive delivery systems for the treatment of chronic inflammatory diseases. Drug Delivery and Translational Research 11: 1475–1497. https://doi.org/10.1007/s13346-021-00977-8. (PMID:33860447).
Fujiwara, N. and K. Kobayashi. Macrophages in inflammation. Current Drug Targets – Inflammation & Allergy 4: 281–286. https://doi.org/10.2174/1568010054022024. (PMID:16101534).
Garzetti L., R. Menon, A. Finardi, A. Bergami, A. Sica, G. Martino, G. Comi, C.Verderio, C. Farina, and R. Furlan. 2014. Activated macrophages release microvesicles containing polarized M1 or M2 mRNAs. Journal of Leukocyte Biology 95: 817–825. https://doi.org/10.1189/jlb.0913485. (PMID: 24379213).
Gentek, R., K. Molawi, and M.H. Sieweke. 2014. Tissue macrophage identity and self-renewal. Immunological Reviews, 262: 56–73. https://doi.org/10.1111/imr.12224. (PMID: 25319327).
Gordon, S. and P.R. Taylor. 2005. Monocyte and macrophage heterogeneity. Nature Reviews Immunology 5: 953–964. https://doi.org/10.1038/nri1733. (PMID: 16322748).
Han, X., S. Ding, H. Jiang, and G. Liu, 2021. Roles of macrophages in the development and treatment of gut inflammation. Frontiers in Cell and Developmental Biology 9: 625423. https://doi.org/10.3389/fcell.2021.625423. (PMID: 33738283).
Healy, D.A., F.A. Wallace, E.A. Miles, and P.C. Calder. 2000. The effect of low to moderate amounts of dietary fish oil on neutrophil lipid composition and function. Lipids 35: 763–768. https://doi.org/10.1007/s11745-000-0583-1. (PMID: 10941877).
Herold, K. and R. Mrowka. 2019. Inflammation-dysregulated inflammatory response and strategies for treatment. Acta Physiologica 226: Article e13284. https://doi.org/10.1111/apha.13284. (PMID: 31009174).
Hinz, B., S.H. Phan, V.J. Thannickal, M. Prunotto, A. Desmoulière, J. Varga, O. De Wever, M. Mareel, and G. Gabbiani. 2012. Recent developments in myofibroblast biology: Paradigms for connective tissue remodeling. The American Journal of Pathology 180: 1340–1355. https://doi.org/10.1016/j.ajpath.2012.02.004. (PMID: 22387320).
Jetten, N., S. Verbruggen, M.J. Gijbels, M.J. Post, M.P. De Winther, and M.M. Donners. 2014. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17: 109–118. https://doi.org/10.1007/s10456-013-9381-6. (PMID: 24013945).
Kim, H.Y., and A.A. Spector. 2018. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Molecular Aspects of Medicine 64: 34–44. https://doi.org/10.1016/j.mam.2018.03.004. (PMID: 29572109).
Kowal, K., R. Silver, E. Sławińska, M. Bielecki, L. Chyczewski, and O. Kowal-Bielecka. 2011. CD163 and its role in inflammation. Folia Histochemica et Cytobiologica 49: 365–374. https://doi.org/10.5603/fhc.2011.0052. (PMID: 22038213).
Krzyszczyk, P., R. Schloss, A. Palmer, and F. Berthiaume. 2018. The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Frontiers in Physiology 9: 419. https://doi.org/10.3389/fphys.2018.00419. (PMID: 29765329).
Latyshev, N.A., E.V. Ermolenko, and S.P. Kasyanov. 2014. Concentration and purification of polyunsaturated fatty acids from squid liver processing wastes. European Journal of Lipid Science and Technology 116: 1608–1613. https://doi.org/10.1002/ejlt.201400083.
Laria, A., A. Lurati, M. Marrazza, D. Mazzocchi, K.A. Re, and M. Scarpellin. 2016. The macrophages in rheumatic diseases. Journal of Inflammation Research 9: 1–11. https://doi.org/10.2147/JIR.S82320. (PMID: 26929657).
Lee, C.H., and E.Y. Choi. 2018. Macrophages and inflammation. Journal of Rheumatic Diseases 25: 11–18. https://doi.org/10.4078/jrd.2018.25.1.11.
Li, H., Y. Meng, S. He, X. Tan, Y. Zhang, X. Zhang, L. Wang, and W. Zheng. 2022. Macrophages, chronic inflammation, and insulin resistance. Cells 11: 3001. https://doi.org/10.3390/cells11193001. (PMID: 36230963).
Li, L., U. Maitra, N. Singh, and L. Gan. 2010. Molecular mechanism underlying LPS-induced generation of reactive oxygen species in macrophages. The FASEB Journal 24: 422.3–422.3. https://doi.org/10.1096/fasebj.24.1_supplement.422.3
Mantovani, A., A. Sica, and M. Locati. 2005. Macrophage polarization comes of age. Immunity 23: 344–346. https://doi.org/10.1016/j.immuni.2005.10.001. (PMID: 16226499).
Mantovani, A., S.K. Biswas, M.R. Galdiero, A. Sica, and M. Locati. 2013. Macrophage plasticity and polarization in tissue repair and remodeling. The Journal of Pathology 229: 176–185. https://doi.org/10.1002/path.4133c. (PMID: 23096265).
McKinney, M.K., and B.F. Cravatt. 2005. Structure and function of fatty acid amide hydrolase. Annual Review of Biochemistry 74: 411–432. https://doi.org/10.1146/annurev.biochem.74.082803.133450. (PMID: 15952893).
Medzhitov, R. 2010. Inflammation 2010: New adventures of an old flame. Cell 140: 771–776. https://doi.org/10.1016/j.cell.2010.03.006. (PMID: 20303867).
Meijerink, J., P. Plastina, J. Vincken, M. Poland, M. Attya, M. Balvers, H. Gruppen, B. Gabriele, and R.F. Witkamp. 2011. The ethanolamide metabolite of DHA, docosahexaenoylethanolamine, shows immunomodulating effects in mouse peritoneal and RAW264.7 macrophages: Evidence for a new link between fish oil and inflammation. British Journal of Nutrition 105: 1798–1807. https://doi.org/10.1017/S0007114510005635. (PMID: 21294934).
Meijerink, J., M. Balvers, and R. Witkamp. 2013. N-acyl amines of docosahexaenoic acid and other n–3 polyunsatured fatty acids – from fishy endocannabinoids to potential leads. British Journal of Pharmacology 169 (4): 772–783. https://doi.org/10.1111/bph.12030.
Meijerink, J., M. Poland, M.G.J. Balvers, P. Plastina, C. Lute, J. Dwarkasing, K. Norren, and R.F. Witkamp. 2014. Inhibition of COX-2-mediated eicosanoid production plays a major role in the anti-inflammatory effects of the endocannabinoid N-docosahexaenoylethanolamine (DHEA) in macrophages. British Journal of Pharmacology 172: 24–37. https://doi.org/10.1111/bph.12747.
Nishi, K., Y. Kanayama, I.H. Kim, A. Nakata, H. Nishiwaki, and T. Sugahara. 2019. Docosahexaenoyl ethanolamide mitigates IgE-mediated allergic reactions by inhibiting mast cell degranulation and regulating allergy-related immune cells. Scientific Reports 9: 16213. https://doi.org/10.1038/s41598-019-52317-z.
Park, T., H. Chen, K. Kevala, J.W. Lee, and H.Y. Kim. 2016. N-Docosahexaenoylethanolamine ameliorates LPS-induced neuroinflammation via cAMP/PKA-dependent signaling. Journal of Neuroinflammation 13: 284. https://doi.org/10.1186/s12974-016-0751-z. (PMID: 27809877).
Paterniti, I., D. Impellizzeri, R. Di Paola, E. Esposito, S. Gladman, P. Yip, J.V. Priestley, A.T. Michael-Titus, and S. Cuzzocrea. 2014. Docosahexaenoic acid attenuates the early inflammatory response following spinal cord injury in mice: In-vivo and in-vitro studies. Journal of Neuroinflammation 11: 6. https://doi.org/10.1186/1742-2094-11-6. (PMID: 24405628).
Porta, C., E. Riboldi, A. Ippolito, and A. Sica. 2015. Molecular and epigenetic basis of macrophage polarized activation. Seminars in Immunology 27: 237–248. https://doi.org/10.1016/j.smim.2015.10.003. (PMID: 26561250).
Romero-Sandoval, A., N. Nutile-McMenemy, and J.A. DeLeo. 2008. Spinal microglial and perivascular cell cannabinoid receptor type 2 activation reduces behavioral hypersensitivity without tolerance after peripheral nerve injury. Anesthesiology 108: 722–734. https://doi.org/10.1097/ALN.0b013e318167af74. (PMID: 18362605).
Rossol, M., H. Heine, U. Meusch, D. Quandt, C. Klein, M.J. Sweet, and S. Hauschildt. 2011. LPS-induced cytokine production in human monocytes and macrophages. Critical Reviews in Immunology 31: 379–446. https://doi.org/10.1615/critrevimmunol.v31.i5.20. (PMID: 22142165).
Rovito, D., C. Giordano, D. Vizza, P. Plastina, I. Barone, I. Casaburi, M. Lanzino, F. Amicis, D. Sisci, L. Mauro, S. Aquila, S. Catalano, D. Bonofiglio, and S. Andò. 2013. Omega-3 PUFA ethanolamides DHEA and EPEA induce autophagy through PPARg activation in MCF-7 breast cancer cells. Journal of Cellular Physiology 228 (6): 1314–1322. https://doi.org/10.1002/jcp.24288. (PMID: 23168911).
Saqib, U., S. Sarkar, K. Suk, O. Mohammad, M.S. Baig, and R. Savai. 2018. Phytochemicals as modulators of M1-M2 macrophages in inflammation. Oncotarget 9: 25; 17937–17950. https://doi.org/10.18632/oncotarget.24788. (PMID: 29707159).
Simon, F., and R. Fernández. 2009. Early lipopolysaccharide-induced reactive oxygen species production evokes necrotic cell death in human umbilical vein endothelial cells. Journal of Hypertension 27: 1202–1216. https://doi.org/10.1097/HJH.0b013e328329e31c. (PMID: 19307985).
Stulnig, T.M., and M. Zeyda. 2004. Immunomodulation by polyunsaturated fatty acids: Impact on T-cell signaling. Lipids 39: 1171–1175. https://doi.org/10.1007/s11745-004-1344-x. (PMID: 15736912).
Sung, J., H. Jeon, I. Kim, H.S. Jeong, and J. Lee. 2017. Anti-inflammatory effects of stearidonic acid mediated by suppression of NF-κB and MAP-kinase pathways in macrophages. Lipids 52: 781–787. https://doi.org/10.1007/s11745-017-4278-6. (PMID: 28744771).
Tang, R., Y.M. Lin, H.X. Liu, and E.S. Wang. 2018. Neuroprotective effect of docosahexaenoic acid in rat traumatic brain injury model via regulation of TLR4/NF-Kappa B signaling pathway. International Journal of Biochemistry and Cell Biology 99: 64–71. https://doi.org/10.1016/j.biocel.2018.03.017. (PMID: 29597004).
Tyrtyshnaia, A., S. Konovalova, A. Bondar, E. Ermolenko, R. Sultanov, and I. Manzhulo. 2021. Anti-inflammatory activity of N-docosahexaenoylethanolamine and N-eicosapentaenoylethanolamine in a mouse model of lipopolysaccharide-induced neuroinflammation. International Journal of Molecular Sciences 22: 10728. https://doi.org/10.3390/ijms221910728.
Tyrtyshnaia, A., S. Konovalova, A. Ponomarenko, A. Egoraeva, and I. Manzhulo. 2022. Fatty acid-derived N-acylethanolamines dietary supplementation attenuates neuroinflammation and cognitive impairment in LPS murine model. Nutrients 14: 3879. https://doi.org/10.3390/nu14183879. (PMID: 36145255).
Ueda, N., K. Tsuboi, and T. Uyama. 2010. N-acylethanolamine metabolism with special reference to N-acylethanolamine-hydrolysing acid amidase (NAAA). Progress in Lipid Research 49: 299–315. https://doi.org/10.1016/j.plipres.2010.02.003. (PMID: 20152858).
Yang, X., Y. Chang, and W. Wei. 2020. Emerging role of targeting macrophages in rheumatoid arthritis: focus on polarization, metabolism and apoptosis. Cell Proliferation 53: (7); Article e12854. https://doi.org/10.1111/cpr.12854. (PMID: 32530555).
Zeidler, P.C., L.M. Millecchia, and V. Castranova. 2004. Role of inducible nitric oxide synthase-derived nitric oxide in lipopolysaccharide plus interferon-gamma-induced pulmonary inflammation. Toxicology and Applied Pharmacology 195 (1): 45–54. https://doi.org/10.1016/j.taap.2003.10.005.
Zhou, D., C. Huang, Z. Lin, S. Zhan, L. Kong, C. Fang, and J. Li. 2014. Macrophage polarization and function with emphasis on the evolving roles of coordinated regulation of cellular signaling pathways. Cellular Signalling 26 (2): 192–197. https://doi.org/10.1016/j.cellsig.2013.11.004.
Funding
This research was funded by the Ministry of Science and Higher Education, Russian Federation (grant 13.1902.21.0012; contract no. 075–15-2020–796).
Author information
Authors and Affiliations
Contributions
Conceptualization: A.E., A.T., and I.M.; methodology: A.T., A.E., I.M., A.P., R.S., and D.I.; validation: A.E. and I.M.; formal analysis: A.E., A.T., and I.M.; investigation: A.T., A.E., I.M., A.P., R.S., and D.I.; resources: A.T., I.M., and R.S.; data curation: I.M.; writing—original draft preparation: A.E., I.M., and A.P.; writing—review and editing: A.E., A.T., and I.M.; visualization: I.M.; supervision: A.T. and I.M.; project administration: A.E. and I.M.; funding acquisition: I.M. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval
All experimental procedures were approved by the Animal Ethics Committee at the A.V. Zhirmunsky National Scientific Center of Marine Biology, Far Eastern Branch, Russian Academy of Sciences (no. 1.3/2022) according to the Laboratory Animal Welfare guidelines and the European Communities Council Directive 2010/63/EU.
Informed Consent
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Egoraeva, A., Tyrtyshnaia, A., Ponomarenko, A. et al. Anti-inflammatory Effect of Polyunsaturated Fatty Acid N-Acylethanolamines Mediated by Macrophage Activity In Vitro and In Vivo. Inflammation 46, 2306–2319 (2023). https://doi.org/10.1007/s10753-023-01879-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-023-01879-2