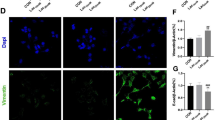
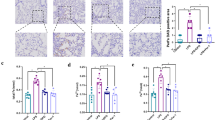
Abstract
Acute lung injury (ALI) is characteristic of the wholesale destruction of the lung endothelial barrier, which results in protein-rich lung edema, influx of pro-inflammatory leukocytes, and intractable hypoxemia, contributing to high mortality. Kindlin-2 is involved in the process of tumor- and wound healing-associated inflammation. However, the effects of kindlin-2 on lipopolysaccharide (LPS)-induced ALI and its mechanisms remain unknown. In this study, we found that the concentration of kindlin-2 was elevated in the lungs of ALI mice. The specific deletion of kindlin-2 by kindlin-2 siRNA attenuated the severity of lung injury, which was demonstrated by the reduced number of pro-inflammatory cells in bronchoalveolar lavage fluid and lung wet/dry weight ratio, and ameliorated pathologic changes in the lungs of ALI mice. Furthermore, kindlin-2 siRNA decreased the mRNA levels of pro-inflammatory factors (IL-1β, IL-6, and TNF-α) and the protein levels of pyroptosis-related proteins. In vitro studies confirmed that LPS + ATP promoted the expressions of pro-inflammatory factors and pyroptosis-related proteins, which was prevented by kindlin-2 siRNA pretreatment in endothelial cells (ECs). In conclusion, inhibition of kindlin-2 developes protective effects against LPS-induced ALI and the cytotoxicity of ECs, which may depend on blocking pyroptosis.





Similar content being viewed by others
Availability of Data and Materials
The data used to support the findings of this study are available from the corresponding author upon request.
References
Liu X, Gao C, Wang Y, Niu L, Jiang S, and S, Pan. 2021. BMSC-derived exosomes ameliorate LPS-induced acute lung injury by miR-384–5p-controlled alveolar macrophage autophagy. Oxidative Medicine and Cellular Longevity 9973457.
Dos Santos, Claudia C., Hajera Amatullah, Chirag M. Vaswani, Tatiana Maron-Gutierrez, Michael Kim, Shirley HJ Mei, Katalin Szaszi et al. 2022. Mesenchymal stromal (stem) Cell (MSC) therapy modulates miR-193b-5p expression to attenuate sepsis-induced acute lung injury. The European Respiratory Journal 59: 2004216.
García-Fernández, Alba, Mónica Sancho, Viviana Bisbal, Pedro Amorós, María D. Marcos, Mar Orzáez, Félix Sancenón, and Ramón Martínez-Máñez. 2021. Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles. A new therapeutic approach for acute lung injury treatment. Journal of Controlled Release: Official Journal of the Controlled Release Society 337: 14–26.
Wang, R., Y. Wang, L. Hu, Z. Lu, and X. Wang. 2021. Inhibition of complement C5a receptor protects lung cells and tissues against lipopolysaccharide-induced injury via blocking pyroptosis. Aging 13: 8588–8598.
Cheng, Kwong Tai, Shiqin Xiong, Zhiming Ye, Zhigang Hong, Anke Di, Kit Man Tsang, and Xiaopei Gao et al. 2017. Caspase-11-mediated endothelial pyroptosis underlies endotoxemia-induced lung injury. The Journal of Clinical Investigation 127: 4124–4135.
Mehta, D., and A.B. Malik. 2006. Signaling mechanisms regulating endothelial permeability. Physiological Reviews 86: 279–367.
Maniatis, N.A., and S.E. Orfanos. 2008. The endothelium in acute lung injury/acute respiratory distress syndrome. Current Opinion in Critical Care 14: 22–30.
Cao, Y., Chen, X., Liu, Y., Zhang, X., Zou, Y., and J. Li. 2021. PIM1 inhibition attenuated endotoxin-induced acute lung injury through modulating ELK3/ICAM1 axis on pulmonary microvascular endothelial cells. Inflammation Research: Official Journal of the European Histamine Research Society [et al] 70: 89–98.
Jorgensen, I., and E.A. Miao. 2015. Pyroptotic cell death defends against intracellular pathogens. Immunological Reviews 265: 130–142.
Tan, S., and S. Chen. 2021. The mechanism and effect of autophagy, apoptosis, and pyroptosis on the progression of silicosis. International Journal of Molecular Sciences 22.
Wang, Y.C., et al. 2019. Dihydromyricetin alleviates sepsis-induced acute lung injury through inhibiting NLRP3 inflammasome-dependent pyroptosis in mice model. Inflammation 42: 1301–1310.
Yang, J., et al. 2016. Hemorrhagic shock primes for lung vascular endothelial cell pyroptosis: role in pulmonary inflammation following LPS. Cell Death & Disease 7: e2363.
Wu, D., et al. 2016. Interferon regulatory factor-1 mediates alveolar macrophage pyroptosis during LPS-induced acute lung injury in mice. Shock (Augusta, Ga) 46: 329–338.
Zou, D.M., Zhou, S.M., Li, L.H., Zhou, J.L., Tang, Z.M., Wang, S.H. 2020. Knockdown of long noncoding RNAs of maternally expressed 3 alleviates hyperoxia-induced lung injury via inhibiting thioredoxin-interacting protein-mediated pyroptosis by binding to miR-18a. The American Journal of Pathology 190: 994–1005.
Noda, K., et al. 2017. Targeting circulating leukocytes and pyroptosis during ex vivo lung perfusion improves lung preservation. Transplantation 101: 2841–2849.
Cao, H., et al. 2020. Focal adhesion protein kindlin-2 regulates bone homeostasis in mice. Bone Research 8: 2.
Guo, L., et al. 2019. Kindlin-2 links mechano-environment to proline synthesis and tumor growth. Nature Communications 10: 845.
Zhu, K., et al. 2020. Kindlin-2 modulates MafA and β-catenin expression to regulate β-cell function and mass in mice. Nature Communications 11: 484.
Godbout, E., et al. 2020. Kindlin-2 mediates mechanical activation of cardiac myofibroblasts. Cells 9.
Rognoni, E., R. Ruppert, and R. Fassler. 2016. The kindlin family: Functions, signaling properties and implications for human disease. Journal of Cell Science 129: 17–27.
Gao. H., et al. 2019. Lipoatrophy and metabolic disturbance in mice with adipose-specific deletion of kindlin-2. JCI Insight 4.
Pan, Y., Wang, Q., Luan, W., Shi, Y., Liu, J., and F. Qi. 2021. Kindlin-2 regulates the differentiation of 3T3-L1 preadipocytes: implications for wound healing. Annals of Translational Medicine 9: 348.
Dong, L., et al. 2021. Activation of TREM-1 induces endoplasmic reticulum stress through IRE-1alpha/XBP-1s pathway in murine macrophages. Molecular Immunology 135: 294–303.
Song, J., et al. 2019. Kindlin-2 inhibits the Hippo signaling pathway by promoting degradation of MOB1. Cell Reports 29: 3664–3677.e3665.
Zhang, P., et al. 2021. Kindlin-2 acts as a key mediator of lung fibroblast activation and pulmonary fibrosis progression. American Journal of Respiratory Cell and Molecular Biology 65: 54–69.
Wei, X., et al. 2013. Kindlin-2 mediates activation of TGF-beta/Smad signaling and renal fibrosis. Journal of the American Society of Nephrology 24: 1387–1398.
Shen, Z., et al. 2013. The novel focal adhesion gene kindlin-2 promotes the invasion of gastric cancer cells mediated by tumor-associated macrophages. Oncology Reports 29: 791–797.
Kurundkar, D., et al. 2019. SIRT3 diminishes inflammation and mitigates endotoxin-induced acute lung injury. JCI Insight 4.
Xu, W.J., et al. 2019. Inhibition of GGPPS1 attenuated LPS-induced acute lung injury and was associated with NLRP3 inflammasome suppression. American Journal of Physiology Lung Cellular and Molecular Physiology 316: L567–l577.
Yan, J., et al. 2018. Nrf2 protects against acute lung injury and inflammation by modulating TLR4 and Akt signaling. Free Radical Biology & Medicine 121: 78–85.
Huang, X.T., et al. 2020. Galectin-1 ameliorates lipopolysaccharide-induced acute lung injury via AMPK-Nrf2 pathway in mice. Free Radical Biology & Medicine 146: 222–233.
Lei, J., et al. 2018. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. European Journal of Pharmacology 818: 110–114.
Wu. X., Kong, Q., Zhan, L., Qiu, Z., Huang, Q., and X. Song. 2019. TIPE2 ameliorates lipopolysaccharide-induced apoptosis and inflammation in acute lung injury. Inflammation Research: Official Journal of the European Histamine Research Society [et al] 68: 981–992.
Frank, D., and J.E. Vince. 2019. Pyroptosis versus necroptosis: Similarities, differences, and crosstalk. Cell Death and Differentiation 26: 99–114.
Shi, J., Gao, W., and F. Shao. 2017. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends in Biochemical Sciences 42: 245–254.
Russo, HM., Rathkey, J., Boyd-Tressler, A., Katsnelson, M.A., Abbott, D.W., and G.R. Dubyak. 2016. Active caspase-1 induces plasma membrane pores that precede pyroptotic lysis and are blocked by lanthanides. Journal of Immunology (Baltimore, Md: 1950) 197: 1353–1367.
Shao, X.F., et al. 2020. Ghrelin alleviates traumatic brain injury-induced acute lung injury through pyroptosis/NF-κB pathway. International Immunopharmacology 79: 106175.
Mitra, S., et al. 2018. Microparticulate caspase 1 regulates gasdermin D and pulmonary vascular endothelial cell injury. American Journal of Respiratory Cell and Molecular Biology 59: 56–64.
Arioz, B.I., et al. 2019. Melatonin attenuates LPS-induced acute depressive-like behaviors and microglial NLRP3 inflammasome activation through the SIRT1/Nrf2 pathway. Frontiers in Immunology 10: 1511.
Acknowledgements
We acknowledge and appreciate our colleagues for their valuable efforts and comments on this paper.
Funding
This work was supported by the High-level introduction of talent research start-up fund (LRYGCC202111, LRYGCC202108), Self-funded scientific research project of Health Commission of Guangxi (Z20210219, Z20200052), and Guangxi Natural Science Foundation (2020GXNSFAA297101).
Author information
Authors and Affiliations
Contributions
Liang Dong and Zhi-Jian You conceived and designed the experiments. Yi-Dan Huang, Yu Fang, Li Ma, and Yi-Qi Zhou performed the experiments. Wen-Long Li, Peng-Jiu Feng, and Yuan-Hao Qin analyzed the data. Liang Dong and Zhi-Jian You contributed reagents/materials/analysis tools. Yi-Dan Huang and Yu Fang wrote the paper. Liang Dong and Zhi-Jian You critically reviewed the manuscript. All authors had final approval of the submitted versions.
Corresponding authors
Ethics declarations
Animal Ethics Approval
All animal experiments were approved by Institutional Animals Care and Use Committee at Liuzhou Municipal People’s Hospital.
Consent for Publication
Not applicable.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, YD., Fang, Y., Ma, L. et al. Kindlin-2 Mediates Lipopolysaccharide-Induced Acute Lung Injury Partially via Pyroptosis in Mice. Inflammation 45, 1199–1208 (2022). https://doi.org/10.1007/s10753-021-01613-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10753-021-01613-w