Abstract
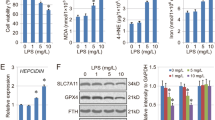
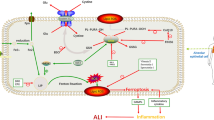
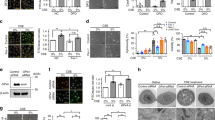
Ferroptosis is a newly proposed form of programmed cell death that is iron-dependent and closely linked to oxidative stress. Its specific morphological changes include shrunken mitochondria, increased density of mitochondrial membrane, and rupture or disappearance of mitochondrial cristae. The main mechanism of ferroptosis involves excessive free iron reacting with membrane phospholipids, known as the Fenton reaction, resulting in lipid peroxidation. However, the role of iron in acute lung injury (ALI) remains largely unknown. In this study, LPS was instilled into the airway to induce ALI in mice. We observed a significant increase in iron concentration during ALI, accompanied by elevated levels of lipid peroxidation markers such as malonaldehyde (MDA) and 4-hydroxynonenal (4-HNE). Treatment with the iron chelator deferoxamine (DFO) or ferroptosis inhibitor ferrostatin-1 (Fer-1) reversed lipid peroxidation and significantly attenuates lung injury. Similarly, DFO or Fer-1 treatment improved the cell survival significantly in vitro. These results demonstrated that ferroptosis occurs during ALI and that targeting ferroptosis is an effective treatment strategy. Interestingly, we found that the increased iron was primarily concentrated in mitochondria and DFO treatment effectively restored normal mitochondria morphology. To further confirm the damaging effect of iron on mitochondria, we performed mitochondrial stress tests in vitro, which revealed that iron stimulation led to mitochondrial dysfunction, characterized by impaired basal respiratory capacity, ATP production capacity, and maximum respiratory capacity. MitoTEMPO, an antioxidant targeting mitochondria, exhibited superior efficacy in improving iron-induced mitochondrial dysfunction compared to the broad-spectrum antioxidant NAC. Treatment with MitoTEMPO more effectively alleviated ALI. In conclusion, ferroptosis contributes to the pathogenesis of ALI and aggravates ALI by impairing mitochondrial function.








Similar content being viewed by others
Data Availability
No datasets were generated or analyzed during the current study.
References
Ranieri, V.M., et al. 2012. Acute respiratory distress syndrome: the Berlin Definition. Journal of the American Medical Association 307 (23): 2526–2533.
Thompson, B.T., R.C. Chambers, and K.D. Liu. 2017. Acute respiratory distress syndrome. New England Journal of Medicine 377 (6): 562–572.
Ashbaugh, D.G., et al. 1967. Acute respiratory distress in adults. Lancet 2 (7511): 319–323.
Bellani, G., et al. 2016. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. Journal of the American Medical Association 315 (8): 788–800.
Stockwell, B.R., et al. 2017. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171 (2): 273–285.
Tang, D., et al. 2021. Ferroptosis: Molecular mechanisms and health implications. Cell Research 31 (2): 107–125.
Stoyanovsky, D.A., et al. 2019. Iron catalysis of lipid peroxidation in ferroptosis: Regulated enzymatic or random free radical reaction? Free Radical Biology & Medicine 133: 153–161.
Liu, Q., et al. 2021. Iron homeostasis and disorders revisited in the sepsis. Free Radical Biology & Medicine 165: 1–13.
Jiang, X., and B.R. Stockwell. 2021. Ferroptosis: Mechanisms, biology and role in disease. Nature Reviews Molecular Cell Biology 22 (4): 266–282.
Camaschella, C., and A. Nai. 2016. Ineffective erythropoiesis and regulation of iron status in iron loading anaemias. British Journal of Haematology 172 (4): 512–523.
Singh, N., et al. 2014. Brain iron homeostasis: From molecular mechanisms to clinical significance and therapeutic opportunities. Antioxidants & Redox Signaling 20 (8): 1324–1363.
Rouault, T.A. 2013. Iron metabolism in the CNS: Implications for neurodegenerative diseases. Nature Reviews Neuroscience 14 (8): 551–564.
Ramm, G.A., and R.G. Ruddell. 2005. Hepatotoxicity of iron overload: Mechanisms of iron-induced hepatic fibrogenesis. Seminars in Liver Disease 25 (4): 433–449.
Zarjou, A., et al. 2013. Proximal tubule H-ferritin mediates iron trafficking in acute kidney injury. The Journal of Clinical Investigation 123 (10): 4423–4434.
Chang, A.L., et al. 2015. Redox regulation of mitophagy in the lung during murine Staphylococcus aureus sepsis. Free Radical Biology & Medicine 78: 179–189.
Terman, A., et al. 2010. Mitochondrial turnover and aging of long-lived postmitotic cells: The mitochondrial-lysosomal axis theory of aging. Antioxidants & Redox Signaling 12 (4): 503–535.
Carré, J.E., et al. 2010. Survival in critical illness is associated with early activation of mitochondrial biogenesis. American Journal of Respiratory and Critical Care Medicine 182 (6): 745–751.
Singer, M. 2014. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 5 (1): 66–72.
Islam, M.N., et al. 2012. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Medicine 18 (5): 759–765.
Kirkham, P.A., and P.J. Barnes. 2013. Oxidative stress in COPD. Chest 144 (1): 266–273.
Meyer, A., et al. 2013. Skeletal muscle mitochondrial dysfunction during chronic obstructive pulmonary disease: Central actor and therapeutic target. Experimental Physiology 98 (6): 1063–1078.
Cloonan, S.M., et al. 2016. Mitochondrial iron chelation ameliorates cigarette smoke-induced bronchitis and emphysema in mice. Nature Medicine 22 (2): 163–174.
Galaris, D., A. Barbouti, and K. Pantopoulos. 2019. Iron homeostasis and oxidative stress: An intimate relationship. Biochimica et Biophysica Acta, Molecular Cell Research 1866 (12): 118535.
Rochette, L., et al. 2022. Lipid peroxidation and iron metabolism: two corner stones in the homeostasis control of ferroptosis. International Journal of Molecular Sciences 24 (1): 449.
Yang, W.S., and B.R. Stockwell. 2016. Ferroptosis: Death by lipid peroxidation. Trends in Cell Biology 26 (3): 165–176.
Liu, J., et al. 2022. Loss of MBD2 ameliorates LPS-induced alveolar epithelial cell apoptosis and ALI in mice via modulating intracellular zinc homeostasis. Federation of American Societies for Experimental Biology Journal 36 (2): e22162.
Chambers, E., S. Rounds, and Q. Lu. 2018. Pulmonary endothelial cell apoptosis in emphysema and acute lung injury. Advances in Anatomy, Embryology and Cell Biology 228: 63–86.
Chopra, M., J.S. Reuben, and A.C. Sharma. 2009. Acute lung injury: Apoptosis and signaling mechanisms. Experimental Biology and Medicine (Maywood, N.J.) 234 (4): 361–371.
Funding
This study was supported by the National Natural Science Foundation of China (82170091, 82130001, 82272243, 81970069, and 82370048); National Key R&D Plan (2020YFC2003700); Shanghai Municipal Science and Technology Major Project (ZD2021CY001); Science and Technology Commission of Shanghai Municipality (20Z11901000, 20DZ2261200, 20XD1401200, and 22Y11900800); Clinical Research Plan of SHDC (SHDC2020CR5010-002); Shanghai Municipal Key Clinical Specialty (shslczdzk02201); Smart Healthcare Project of Zhongshan Hospital, Fudan University (project number 2020ZHZS18); Youth Science Project of Zhongshan Hospital, Fudan University (2023ZSQN21); and Shanghai Municipal Health Commission and Shanghai Municipal Administrator of Traditional Chinese Medicine (ZY(2021-2023)-0207-01).
Author information
Authors and Affiliations
Contributions
Yuanlin Song, Dong Yang, and Linlin Wang contributed to the design of the study; Xiaocen Wang, Tingting Wei, and Jinlong Luo performed the in vivo experiment; Ke Lang, Zhaolin Gu, and Xinyi Ning performed the in vitro experiment; Xiaocen Wang, Tingting Wei, and Jinlong Luo performed the data analyses and wrote the manuscript; Yansha Song, Cuicui Chen, and Yencheng Chao helped perform the analysis with constructive discussions. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Conflict Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Wei, T., Luo, J. et al. Iron Overload–Dependent Ferroptosis Aggravates LPS-Induced Acute Lung Injury by Impairing Mitochondrial Function. Inflammation (2024). https://doi.org/10.1007/s10753-024-02022-5
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10753-024-02022-5