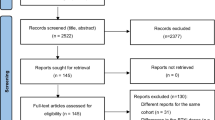
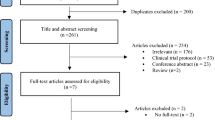
Abstract
BTKi is an effective treatment in chronic lymphocytic leukemia. However, head-to-head clinical trials between BTKi are rare. To explore evidence-based treatment decisions, we conducted this network meta-analysis. We searched in PubMed, Cochrane Library and Embase and selected articles of BTKi treatment in CLL patients, with English restrictions. Objective response rate (ORR), progression-free survival (PFS) and safety were outcomes. Combination therapy and acalabrutinib monotherapy achieved great ORR (greater than 80%). Combination therapy (AO and IR) also performed terrific PFS (> 80%). Compared with ibrutinib monotherapy, zanubrutinib, acalabrutinib and IR showed no significance in overall survival. Diarrhea, hypertension, cardiac events, neutropenia were common adverse events of BTKi therapy. IR had higher incidence of hypertension (0.38, 95% CI 0.28–0.48), and IU was more likely occurred cardiac events. Zanubrutinib monotherapy had lower incidence of total serious adverse reaction (0.42, 95% confidence interval (95% CI): 0.36–0.47),while ibrutinib monotherapy occurred higher adverse reactions of grade ≥ 3 (0.77, 95% CI 0.72–0.82). Although both BTKi monotherapy and combination therapy showed great efficacy, combination therapy did not display priority. Meanwhile, safety of BTKi combination therapy needs to be fully and comprehensively considered.
Registration number: CRD42022378732.









Similar content being viewed by others
Data availability
All data could be obtained from corresponding author.
Change history
02 December 2023
A Correction to this paper has been published: https://doi.org/10.1007/s10238-023-01251-6
Abbreviations
- AO:
-
Acalabrutinib plus Obinutuzumab
- IU:
-
Ibrutinib plus Ublituximab
- IR:
-
Ibrutinib plus Rituximab
- Aca:
-
Acalabrutinib
- Ibr:
-
Ibrutinib
- Zan:
-
Zanubrutinib
- PFS:
-
Progression-free survival
- CLL:
-
Chronic lymphocytic leukemia
- SLL:
-
Small lymphocytic lymphoma
- BTKi:
-
Bruton’s tyrosine kinase inhibitors
- ORR:
-
Objective response rate
- PFS:
-
Progression-free survival
- OS:
-
Overall survival
- AEs:
-
Adverse events
- TEAEs:
-
Treatment-emergent adverse events
- SAE:
-
Serious adverse events
- NMA:
-
Network meta-analysis
- iwCLL:
-
International Workshop on CLL
- RCTs:
-
Randomized controlled trials
- ECOG:
-
Eastern Cooperative Oncology Group
- 95%CI:
-
95% confidence interval
- SUCRA:
-
Surface Under Cumulative Ranking
- ECOG:
-
Eastern Cooperative Oncology Group
- HR:
-
Hazard ratio
References
Hallek M, Cheson BD, Catovsky D, et al. iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood. 2018;131(25):2745–60. https://doi.org/10.1182/blood-2017-09-806398.
Ahn IE, Farooqui M, Tian X, et al. Depth and durability of response to ibrutinib in CLL: 5-year follow-up of a phase 2 study. Blood. 2018;131(21):2357–66. https://doi.org/10.1182/blood-2017-12-820910.
Hallek M, Fischer K, Fingerle-Rowson G, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, open-label, phase 3 trial. Lancet. 2010;376(9747):1164–74. https://doi.org/10.1016/S0140-6736(10)61381-5.
Pal SS, Dammeijer F, Hendriks RW. Role of Bruton’s tyrosine kinase in B cells and malignancies. Mol Cancer. 2018;17(1):57. https://doi.org/10.1186/s12943-018-0779-z.
Garg N, Padron EJ, Rammohan KW, et al. Bruton's Tyrosine Kinase Inhibitors: the next frontier of b-cell-targeted therapies for cancer, autoimmune disorders, and multiple sclerosis. J Clin Med. 2022;11(20). https://doi.org/10.3390/jcm11206139
Xu W, Yang S, Tam CS, et al. Zanubrutinib monotherapy for Naïve and relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma: a pooled analysis of three studies. Adv Ther. 2022;39(9):4250–65. https://doi.org/10.1007/s12325-022-02238-7.
Allan JN, Shanafelt T, Wiestner A, et al. Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials. Br J Haematol. 2022;196(4):947–53. https://doi.org/10.1111/bjh.17984.
Jones J, Mato A, Coutre S, et al. Evaluation of 230 patients with relapsed/refractory deletion 17p chronic lymphocytic leukaemia treated with ibrutinib from 3 clinical trials. Br J Haematol. 2018;182(4):504–12. https://doi.org/10.1111/bjh.15421.
Byrd JC, Furman RR, Coutre SE, et al. Ibrutinib treatment for first-line and relapsed/refractory chronic lymphocytic leukemia: final analysis of the pivotal phase Ib/II PCYC-1102 study. Clin Cancer Res. 2020;26(15):3918–27. https://doi.org/10.1158/1078-0432.CCR-19-2856.
Sharman JP, Egyed M, Jurczak W, et al. Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzmab for treatment-naive chronic lymphocytic leukaemia (ELEVATE TN): a randomised, controlled, phase 3 trial Lancet, 2020;395(10232):1278–91. https://doi.org/10.1016/S0140-6736(20)30262-2
Kahl BS, Giannopoulos K, Jurczak W, et al. CLL-137 SEQUOIA: results of a phase 3 randomized study of Zanubrutinib Versus Bendamustine + Rituximab (BR) in patients with treatment-Naïve (TN) chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/ SLL). Clin Lymphoma Myeloma Leuk. 2022;22(Suppl 2):S269–70.
Sharman JP, Brander DM, Mato AR, et al. Ublituximab plus ibrutinib versus ibrutinib alone for patients with relapsed or refractory high-risk chronic lymphocytic leukaemia (GENUINE): a phase 3, multicentre, open-label, randomised trial. Lancet Haematol. 2021;8(4):e254–66. https://doi.org/10.1016/S2352-3026(20)30433-6.
Burger JA, Sivina M, Jain N, et al. Randomized trial of ibrutinib vs ibrutinib plus rituximab in patients with chronic lymphocytic leukemia. Blood. 2019;133(10):1011–9. https://doi.org/10.1182/blood-2018-10-879429.
Hillmen P, Eichhorst B, Brown JR, et al. Zanubrutinib versus ibrutinib in relapsed/refractory chronic lymphocytic leukemia and small lymphocytic lymphoma: interim analysis of a randomized phase III trial. J Clin Oncol. 2023;41(5):1035–45. https://doi.org/10.1200/JCO.22.00510.
Morche J, Freitag S, Hoffmann F, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. Z Evid Fortbild Qual Gesundhwes, 2020,150–152:124–133. https://doi.org/10.1016/j.zefq.2019.11.003
Cull G, Burger JA, Opat S, et al. Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: long-term follow-up of the phase I/II AU-003 study. Br J Haematol. 2022;196(5):1209–18. https://doi.org/10.1111/bjh.17994.
Burger JA, Tedeschi A, Barr PM, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med. 2015;373(25):2425–37. https://doi.org/10.1056/NEJMoa1509388.
Woyach JA, Ruppert AS, Heerema NA, et al. Ibrutinib regimens versus chemoimmunotherapy in older patients with untreated CLL. N Engl J Med. 2018;379(26):2517–28. https://doi.org/10.1056/NEJMoa1812836.
Byrd JC, Hillmen P, Ghia P, et al. Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial. J Clin Oncol. 2021;39(31):3441–52. https://doi.org/10.1200/JCO.21.01210.
Brown JR, Eichhorst B, Hillmen P, et al. Zanubrutinib or Ibrutinib in relapsed or refractory chronic lymphocytic leukemia[J]. N Engl J Med. 2023;388(4):319–32. https://doi.org/10.1056/NEJMoa2211582
Danilov AV, Herbaux C, Walter HS, et al. Phase Ib study of tirabrutinib in combination with idelalisib or entospletinib in previously treated chronic lymphocytic leukemia. Clin Cancer Res. 2020;26(12):2810–8. https://doi.org/10.1158/1078-0432.CCR-19-3504.
Davids MS, Waweru C, Le Nouveau P, et al. Comparative efficacy of acalabrutinib in frontline treatment of chronic lymphocytic leukemia: a systematic review and network meta-analysis. Clin Ther. 2020;42(10):1955–74. https://doi.org/10.1016/j.clinthera.2020.08.017.
Chen P H, Ho C L, Lin C, et al. Treatment outcomes of novel targeted agents in relapse/refractory chronic lymphocytic leukemia: a systematic review and network meta-analysis. J Clin Med, 2019,8(5). https://doi.org/10.3390/jcm8050737
Acknowledgements
Yi Fang, Jin Lu and Qian Lu contributed to study design and guidance support. Liming Chen and Nan Peng selected eligible studies. Binyi Hu, Dingyuan Hu and Jiaojiao Zhang extracted and analyzed the data from original articles. Lijue Wang and Zhenwei Xie made the charts and interpreted the results. Suping Niu and Xiangxing Liu interpreted the results and wrote the manuscript. Xiangxing Liu contributed to review and revised the manuscript. All authors read and approved the final version of the manuscript. Thanks to all the authors for the efforts of this study.
Funding
This study was funded by the project “Demonstration construction project of research ward (4102000007).”
Author information
Authors and Affiliations
Contributions
Yi Fang, Jin Lu and Qian Lu contributed to study design and guidance support. Liming Chen and Nan Peng selected eligible studies. Binyi Hu, Dingyuan Hu and Jiaojiao Zhang extracted and analyzed the data from original articles. Lijue Wang and Zhenwei Xie made the charts and interpreted the results. Suping Niu and Xiangxing Liu interpreted the results and wrote the manuscript. Xiangxing Liu contributed to review and revised the manuscript. All authors read and approved the final version of the manuscript. Thanks to all the authors for the efforts of this study.
Corresponding authors
Ethics declarations
Conflict of interest
We declare that we have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. All authors have no relevant financial or non-financial interests to disclose.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: Missing co-author names have been updated.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Hu, B., Peng, N. et al. Evaluation of Bruton tyrosine kinase inhibitors monotherapy and combination therapy in lymphocytic leukemia. Clin Exp Med 23, 4237–4248 (2023). https://doi.org/10.1007/s10238-023-01208-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-023-01208-9