Abstract
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a type of mature B lymphocyte clonal proliferative tumor with a specific immunophenotype. Bruton tyrosine kinase inhibitors (BTKi) have been approved for the treatment of CLL/SLL. However, the efficacy and safety of new-generation BTKi-based regimens have not been systematically studied. In this systematic review, we evaluated the efficacy and safety of new-generation BTKi-based regimens for the treatment of patients with CLL/SLL. A comprehensive search on PubMed, Embase, Cochrane Library, and ClinicalTrials.gov. up to January 31, 2023, was conducted by us. Studies reporting data on CLL/SLL patients treated with new-generation BTKi were included. We assessed the overall response rate (ORR), complete response (CR) rate, and 24-month OS/PFS rates for efficacy analysis. For safety analysis, we evaluated the incidence of grade ≥ 3 adverse events (AEs). The meta-analysis included twenty studies. The pooled ORR for new-generation BTKi was 92% (95% CI, 89–95%, I2 = 80.68%, P = 0.00), while the pooled CR rate was 10% (95% CI, 6–14%, I2 = 88.11%, P = 0.00). Research has found that the new-generation BTKi-based therapy had higher efficacy under the following treatment conditions: < 65 years old, treatment-naive (TN)-CLL, and BTKi combination therapy. The ORR/CR rates and 24-month OS/PFS rates of BTKi combination therapy were higher than that of BTKi monotherapy. Compared to acalabrutinib monotherapy, zanubrutinib monotherapy demonstrated higher ORR/CR rates and 24-month OS/PFS rates. Common grade ≥ 3 AEs included cytopenia and hypertension. The new-generation BTKi-based therapy has good tolerance and provides incremental benefits for CLL/SLL patients. Despite the superior efficacy of BTKi combination therapy compared to monotherapy, its AEs rates are relatively high. Compared to acalabrutinib, Zanubrutinib may be the preferred monotherapy for CLL. However, randomized-controlled studies are still needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) is a kind of mature B-lymphocyte clonal proliferative tumor characterized by a specific immunophenotype. It is characterized by the accumulation of lymphocytes in peripheral blood, bone marrow, lymph nodes, and spleen, and primarily affects middle-aged and elderly individuals [1]. As the most common leukemia in adults in Western countries, CLL accounts for approximately 25–35% of all leukemias in the USA. This disease is more common among white Americans and is predominantly male. The median age at diagnosis is approximately 72 years old. In 2023, an estimated 18,740 people will be diagnosed with CLL/SLL in the USA, and an estimated 4490 people will die from this disease[2,3]. CLL is a highly heterogeneous disease. Over the past two decades, a major focus of pharmacologic research was signaling through the B-cell receptor (BCR). Several BCR-targeted agents have been approved for use in CLL patients, including Bruton tyrosine kinase (BTK) inhibitors.
In the USA, the Food and Drug Administration (FDA) has approved Ibrutinib, which is a first-in-class BTKi that irreversibly binds to BTK, for the treatment of CLL.[4]. Ibrutinib offers a chemotherapy-free treatment option initially explored in relapsed or refractory (R/R) CLL/SLL patients. The PCYC-1103 study established 420 mg as the RP2D and the RESONATE trial proved that ibrutinib is superior to anti-CD20 ofatumumab[5,6]. With continued research, treatment-related adverse events such as bleeding, diarrhea, and cardiovascular toxicity have attracted attention. Binding of ibrutinib to additional kinases (e.g., EGFR TEC ITK) may potentially contribute to these side effects, prompting development of new-generation BTKi that demonstrate more specific and sustained effects compared to first-generation BTKi in in vivo and preclinical models [7]. The new-generation BTKi currently used to treat CLL include acalabrutinib, zanubrutinib, and tirabrutinib which have higher BTK selectivity and lower/no inhibitory effect on EGFR, TEC, ITK, etc.
We conducted a comprehensive search of CLL studies related to next-generation BTKi treatment in this meta-analysis study and analyzed efficacy and safety data. In addition, we further evaluated the efficacy of BTKi treatment through subgroup analysis, aiming to provide compelling evidence for more rational and effective application of BTKi.
Methods
Registration and protocol
The systematic review protocol was registered on the PROSPERO under CRD42023398266.
Search strategy
We carefully searched PubMed, Embase, the Cochrane Library, and ClinicalTrials.gov to identify relevant studies published up to January 31, 2023, using subject words combined with free words. The subject words were “Leukemia, Lymphocytic, Chronic, B-Cell” and “Bruton Tyrosine Kinase Inhibitor, acalabrutinib, zanubrutinib, orelabrutinib, tirabrutinib”; Supplementary Table S1 provides detailed search terms used in different databases. To ensure a comprehensive search and evaluation of all potentially relevant studies, the search will not be limited by region, race, or age. In addition, the list of references for the identified articles and comments has been rigorously checked by us.
Inclusion and exclusion criteria
The inclusion criteria: (1) prospective clinical studies (including single-arm studies and randomized control trials); (2) studies including patients diagnosed with CLL/SLL; (3) studies involving patients treated with new-generation BTKi (acalabrutinib, zanubrutinib, orelabrutinib, or tirabrutinib), both as single-agent therapy and in combination with other agents; (4) studies reporting both efficacy and safety endpoints, including the overall response rate (ORR), complete response (CR) rate, and adverse events (AEs); (5) published in English and related to human clinical trial.
The exclusion criteria: (1) differences in the doses assigned to patients; (2) significant flaws in statistical methods or experimental design; (3) the repeated publication or similar research; (4) reported outcomes treated by ibrutinib; (5) article type: conference abstract, comment, letters, review, and case report; (6) reported incomplete information; (7) cell or animal study.
Data extraction
All relevant studies were imported into Endnote 9.1 software, and duplicates were subsequently removed. Two reviewers independently extract duplicate data based on inclusion and exclusion criteria (YS, ZXH) using a self-designed data collection form. In case of any discrepancies or disagreements, by consulting the third author (CF) or consensus-based discussion.
The following information was extracted from each study: (1) general characteristics of each study (first author’s name, publication year, ClinicalTrials.gov Identifier, phase of the study); (2) descriptive data of the patients (number of patients, median age, disease status); (3) treatment strategies and the dose of BTKi; (4) primary efficacy endpoints (ORR, CRR) and secondary efficacy endpoints (24-month OS/PFS rate); (5) the number of grade ≥ 3 AEs.
Study outcome evaluation
The definition of ORR was the proportion of patients with CR, CR with incomplete hematological recovery, nodular partial response (PR), PR with lymphocytosis, or PR. The CR rate included CR and eliminated CR with incomplete hematological recovery. Response assessments were conducted for CLL per the “International Workshop on Chronic Lymphocytic Leukemia” (IWCLL) 2008 criteria[8], and for SLL per the Lugano classification for lymphoma 2014[9]. Besides, “the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03” will be used to classify different types of AEs. PFS was defined as the time from the randomization date to progressive disease or death from any cause, and OS was calculated as the date from the random assignment until death due to any cause.
Quality and risk of bias assessment
The methodological quality and risk of bias of included randomized controlled trials were assessed using the Cochrane risk-of-bias tool[10]. The methodological index for non-randomized studies (MINORS) was used to assess prospective single-arm studies.[11].
Statistical analysis
All data analyses were performed using the STATA SE15.1 (StataCorp, TX, USA). For new-generation BTKi-based regime efficacy, we calculated the pooled ORR and CR rate, with 95% confidence intervals (CI). The Cochrane’s Q chi-square test and I2 statistic were used to examine the heterogeneity across studies. P < 0.05 was considered statistically significant. The fixed-effects model was used for pooled results with low heterogeneity (I2 ≤ 50% and/or P ≥ 0.10); otherwise, the random-effects model was used for analysis. By excluding each study one by one from the pooled results with high heterogeneity, sensitivity analysis was performed. To explore the potential impact of different factors on the measurement of results, the sub-group analysis will be conducted on variables including age (< 65 vs. ≥ 65 years), disease status (TN-CLL vs. RR-CLL), treatment strategy (monotherapy vs. combination therapy), monotherapy (acalabrutinib vs. zanubrutinib). For safety, we calculated the toxicity rate similarly, with 95% confidence intervals, and the subgroup analysis by monotherapy was applied. Publication bias was assessed using Begg’s and Egger’s tests. Significant publication bias was defined as a P value < 0.05.
Results
Study selection and characteristics
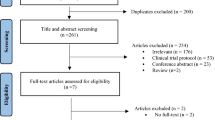
In our search, a total of 3363 records were retrieved, including 841 duplicate reports and 2522 records that underwent title and abstract review. A total of 2377 records were excluded due to the following reasons: other diseases (n = 41), other drugs (n = 361), conference abstract (n = 1278), case reports (n = 21), reviews (n = 533), meta-analyses (n = 8), note (n = 29), letter (n = 24), cell/animal studies (n = 24), and National Clinical Trial registration (n = 58). For 145 records, the full text was reviewed, and 130 of them were excluded based on following reasons: different reports for the same cohort (n = 31), review (n = 24), updated results (n = 31), no reporting of the primary outcome (n = 15), differences in the BTKi doses (n = 22) and other reasons (n = 7). Ultimately, our meta-analysis included 15 records. Figure 1 showed the literature and identification process. The meta-analysis evaluated the efficacy and safety of the new-generation BTKi for a total of 2066 CLL/SLL patients, across ten single-arm studies and five randomized studies. Considering that some records have more than one disease state or intervention, we divide them into 20 studies. Table 1 summarized the baseline clinical characteristics of these patients.
Quality assessment
Ten single-arm studies assessed using the MINORS index score ranged from 12 to 22 points, which was acceptable for the present meta-analysis [Table 2]. Five RCTs were independently evaluated for quality using the Cochrane Collaboration risk of bias tool [Supplementary Figure S1].
Efficacy
Tumor response
All 20 studies reported ORR and CR rates as clinical outcomes. The pooled ORR for new-generation BTKi was 92% (95% CI, 89–95%, I2 = 80.68%, P = 0.00), while the pooled CR rate was 10% (95% CI, 6–14%, I2 = 88.11%, P = 0.00) [Fig. 2]. Analysis using the random-effects model confirmed the considerable efficacy of new-generation BTKi treatment in CLL.
Sub-group analysis on age
All 20 studies evaluated the ORR and CR rates for the age group. The younger patients (< 65 years old) appeared to have higher ORR (95%, 95% CI, 88–99%) than older patients (≥ 65 years old) (90%, 95% CI, 86–93%). Both groups exhibited heterogeneity (I2 = 79.48%, P = 0.00 for the younger, and I2 = 80.44%, P = 0.00 for the older), so the random-effect model was used. The respective CR rate for the younger and older younger groups were 16% (95% CI, 5–30%, I2 = 90.03%, P = 0.00) and 6% (95% CI, 4–10%, I2 = 82.97%, P = 0.00). The pooled results showed that the younger group has better efficacy. [Supplementary Figure S2].
Sub-group analysis on disease status
All 20 studies included the ORR and CR rates of BTKi therapy by disease status (TN-CLL vs RR-CLL). Statistical analysis of TN CLL patients showed that the pooled ORR was 96% (95% CI, 92–98%, I2 = 74.37%, P = 0.00), while the pooled CR rate was 16% (95% CI, 7–28%, I2 = 93.53%, P = 0.00). Five studies reported the ORR and CR rate of R/R CLL. The pooled ORR and CR rate were 90% (95% CI, 85–95%, I2 = 77.17%, P = 0.00) and 7% (95% CI, 4–10%, I2 = 56.68%, P = 0.04) respectively. The pooled outcomes indicated better efficacy in the TN-CLL group [Supplementary Figure S3].
Sub-group analysis on treatment strategy
Sub-group analysis of new-generation BTKi combined with different treatment measures demonstrated that the pooled ORR and CR rate varied among different treatment strategies. BTKi monotherapy was given in sixteen studies, and the pooled ORR was 90% (95% CI, 88–91%, I2 = 80.88%, P = 0.00). Acalabrutinib monotherapy was reported in eight studies, and the pooled ORR was 87% (95% CI, 81–93%, I2 = 82.23%, P = 0.00). Seven studies used zanubrutinib monotherapy for CLL patients, and the pooled ORR was 93% (95% CI, 89–97%, I2 = 79.48%, P = 0.00). Pooled CR rate in BTKi monotherapy was 7% (95% CI, 4–12%, I2 = 85.85%, P = 0.00), which was 3% (95% CI, 1–6%, I2 = 61.78%, P = 0.00) and 13% (95% CI, 6–22%, I2 = 90.36%, P = 0.00) in acalabrutinib and zanubrutinib sub-group respectively. Only one study evaluated tirabrutinib, and the pooled ORR was 83% (95% CI, 64–94%), while the pooled CR rate was 7% (95% CI, 1–23%).
Moreover, four studies with 261 patients assessed BTKi combination (acalabrutinib combination) therapy, and the pooled ORR was 97% (95% CI, 94–99%, I2 = 46.41%, P = 0.13), while the pooled CR rate was 22% (95% CI, 8–42%, I2 = 86.09%, P = 0.00). Twelve studies reported the ORR and CR rate of acalabrutinib-based regimens. The pooled ORR and CR rate were 91% (95% CI, 86–95%, I2 = 83.18%, P = 0.00) and 8% (95% CI, 4–14%, I2 = 87.81%, P = 0.00) respectively [Supplementary Figure S4, S5, and S6].
Survival
Several studies reported survival data for the 24-month OS rate and 24-month PFS rate. Pooled 24-month OS rate for CLL patients treated with BTKi was 94% (95% CI, 92–97%, I2 = 51.32%, P = 0.06). Sub-group analysis for the acalabrutinib monotherapy and zanubrutinib monotherapy showed a pooled 24-month OS rate of 92% (95% CI, 89–96%, I2 = 0.00%) and 95% (95% CI, 92–96%, I2 = 0.00%, P = 0.72), respectively [Supplementary Figure S7]. Pooled 24-month PFS rate for CLL patients treated with BTKi was 86% (95% CI, 82–90%, I2 = 72.16%, P = 0.00).
Sub-group analysis for the acalabrutinib monotherapy and zanubrutinib monotherapy showed a pooled 24-month PFS rate of 83% (95% CI, 75–90%, I2 = 57.74%, P = 0.05) and 86% (95% CI, 80–91%, I2 = 77.84%, P = 0.00), respectively [Supplementary Figure S8]. Sub-group analysis for the BTKi combination therapy and BTKi monotherapy showed a pooled 24-month OS rate of 96% (95% CI, 93–99%, I2 = 0.00%) and 93% (95% CI, 90–96%, I2 = 61.88%, P = 0.03), respectively. The pooled 24-month PFS rate for BTKi combination therapy was 94% (95% CI, 90–97%, I2 = 0.00%), while for BTKi monotherapy was 85% (95% CI, 80–89%, I2 = 65.28%, P = 0.00) [Supplementary Figure S9]. When comparing survival according to disease status, TN patients had a higher pooled 24-month OS rate (95% vs. 82%), and 24-month PFS rate (89% vs. 77%) compared to R/R patients [Supplementary Figure S10].
Immunoglobulin heavy-chain variable gene (IGHV) status
Four studies reported the ORR of IGHV status. The fixed-effects model meta-analysis (I2 = 28.1%, P = 0.249) demonstrated that there was no statistically significant difference in ORR between unmutated IGHV and mutated IGHV (RR = 1.10, 95%CI, 0.99–1.21, P = 0.07) [Supplementary Figure S11].
Toxicity
AEs were reported in all studies; neutropenia, anemia, and thrombocytopenia are the main hematological AEs. The pooled rate of grade ≥ 3 neutropenia was 17% (95% CI, 13–22%, I2 = 83.49%, P = 0.00), grade ≥ 3 anemia was 4% (95% CI, 2–7%, I2 = 83.01%, P = 0.00), and grade ≥ 3 thrombocytopenia was 5% (95% CI, 3–8%, I2 = 79.10%, P = 0.00). Severe non-hematological AEs mainly included diarrhea, fatigue, upper respiratory tract infection, atrial fibrillation, and hypertension. The pooled rate of grade ≥ 3 diarrhea was 1% (95% CI, 1–2%, I2 = 24.68%, P = 0.18), while the pooled rate of grade ≥ 3 fatigue was 1% (95% CI, 1–2%, I2 = 0.00%, P = 0.93). The pooled rate of grade ≥ 3 upper respiratory tract infection was 1% (95% CI, 0–2%, I2 = 51.00%, P = 0.01). The pooled rate of grade ≥ 3 atrial fibrillation and hypertension was 1% (95% CI, 1–2%, I2 = 43.06%, P = 0.03) and 4% (95% CI, 2–7%, I2 = 76.57%, P = 0.00), respectively [Supplementary Figure S12; Table 3]. Table 4 illustrated the pooled rates of grade ≥ 3 AEs in both BTKi monotherapy and BTKi combination therapy. The pooled rates of grade ≥ 3 upper respiratory tract infection and atrial fibrillation were both 1%. BTKi monotherapy exhibited a higher pooled rate of grade ≥ 3 hypertension (5% vs. 2%) compared to BTKi combination therapy. Conversely, the pooled rates of other grade ≥ 3 AEs were consistently lower in BTKi monotherapy when compared to BTKi combination therapy. Table 5 demonstrated the pooled results of grade ≥ 3 AEs between acalabrutinib and zanubrutinib monotherapy. The pooled rates of grade ≥ 3 neutropenia, anemia, and thrombocytopenia in acalabrutinib monotherapy were 14%, 7%, and 5% respectively. The pooled rates of grade ≥ 3 neutropenia, anemia, and thrombocytopenia in zanubrutinib monotherapy were 19%, 2%, and 4% respectively. Zanubrutinib monotherapy had a similar pooled rate of grade ≥ 3 upper respiratory tract infection (2% vs. 1%), and grade ≥ 3 hypertension (6% vs. 4%) compared to acalabrutinib monotherapy. The pooled rates of other grade ≥ 3 AEs were both 1%.
Analysis of publication bias
In this study, Egger’s and Begg’s tests were conducted on ORR and CR rate to determine publication bias Table 6. It was regarded as no publication bias if the P value > 0.05 was met in both methods. The pooled ORR assessment results did not show significant publication bias among included studies. For CR rate, publication bias occurred in the total cohort, TN-CLL, and acalabrutinib-based groups. No publication bias was found in the Egger’s and Begg’s tests for AEs (grade ≥ 3) regarding safety outcomes. The funnel chart of Egger’s and Begg’s is shown partly included in Fig. 3.
Sensitivity analysis
Sensitivity analysis was conducted by removing individual studies from highly heterogeneous aggregated results one by one. When omitting the study, the pooled analysis of ORR, CR rate, and grade ≥ 3 AE did not show significant changes, indicating that our comprehensive results are reliable. Some of the results are shown in Fig. 4.
The graph of sensitivity analysis. A The sensitivity analysis of the total overall response rate; B the sensitivity analysis of the total complete response rate; C the sensitivity analysis of total grade ≥ 3 thrombocytopenia rate; D the sensitivity analysis of total grade ≥ 3 atrial fibrillation rate
Discussion
Chronic lymphocytic leukocytosis (CLL) is a B-cell malignant tumor characterized by the clonal aggregation of CD5 + and CD19 + B cells in the bone marrow and peripheral blood. The World Health Organization (WHO) classifies CLL as an indolent B-cell lymphoma [27]. The prognosis of patients with CLL is very heterogeneous, some patients have an inert course of disease and do not need treatment for life, while others invade the course of disease, showing early treatment indications. Until recently, chemoimmunotherapy with fludarabine, rituximab, and cyclophosphamide, rituximab and bendamustine, or chlorambucil and obinutuzumab was the standard care for TN patients who were physically fit (fludarabine, rituximab, and cyclophosphamide) or had coexisting conditions (chlorambucil plus bendamustine/obinutuzumab and rituximab). The only way to cure CLL is allogeneic hematopoietic stem cell transplantation, but unfortunately, most patients are not suitable for transplantation and can only manage the disease and symptoms with drugs.
The BCR signaling pathway is involved in the pathogenesis of CLL. There is no doubt that the BCR signaling pathway is essential for maintaining the survival, proliferation, and development of CLL cells. Drugs inhibiting the enzymes involved in the BCR pathway, specifically BTK, are the standard care for treating CLL nowadays. Ibrutinib is the world’s first covalent, irreversible BTKi approved by the US FDA. RESONATE[28], RESONATE-2[29], and ECOG 1912[30] respectively established the efficacy of ibrutinib in R/R CLL, TN CLL ≥ 65 years old, and TN CLL ≤ 70 years old. A recent pooled analysis of four clinical trials showed that in TN CLL with TP53 aberrations, the 4-year PFS rate was 79% and the 4-year total survival (OS) rate was 88%[31]. Therefore, ibrutinib is recommended as the preferred treatment for CLL patients with TP53 aberrations. With the prolongation of survival brought by the continuous optimization of treatment, the proportion of elderly patients with CLL increases, while elderly patients are often complicated with cardiovascular and cerebrovascular diseases, hypertension, diabetes, and so on. In this context, clinical attention to the safety of treatment is increasingly important. The safety results of the 8-year follow-up of RESONATE-2 showed that the incidence of hypertension was more than 20% and the incidence of atrial fibrillation was about 10% in the last 3 years[32]. Additionally, the FDA updated the manual of ibrutinib to warn of cardiac safety issues in May 2022. Fatal and serious cardiac failure and cardiac arrhythmias have occurred after ibrutinib administration. Among 4896 patients who underwent clinical trials of ibrutinib (including monotherapy or combination therapy), 1% of patients died from cardiac causes or sudden death. These AEs occurred in patients both with and without preexisting hypertension or cardiac comorbidities. Patients with cardiac comorbidities may have a greater risk[33]. Considering the toxicity of ibrutinib, it has been moved from “Preferred regimens” to “Other recommended regimens” in the National Comprehensive Cancer Network Guidelines (NCCN Guidelines Version 1.2023) for CLL. Patients’ cardiovascular function must be strictly evaluated before using ibrutinib[34].
Acalabrutinib, zanubrutinib, and tirabrutinib are new covalent BTKis that exhibit greater selectivity for BTK compared to ibrutinib and were initially anticipated to have a more favorable safety profile. In the phase II clinical trial, 33 patients with CLL who were intolerant to ibrutinib were treated with acalabrutinib, 72% had no recurrent adverse reactions related to ibrutinib, and 13% had adverse reactions related to ibrutinib, but the degree was reduced. With a median follow-up of 19 months, ORR was 76% including 1 patient who achieved CR[14]. SEQUOIA [23]study evaluated the efficacy and safety of zanubrutinib versus bendamustine plus rituximab (BR) in the first-line treatment of elderly or young CLL patients with comorbidities without del (17p). At a median follow-up of 26.2 months, the 24-month PFS rate assessed by IRC was 85.5% in the zanubrutinib group and 69.5% in the BR group. PFS was significantly improved in the zanubrutinib group compared with the BR group (HR = 0.42, two-sided P < 0.0001). Subgroup analysis showed that regardless of age, gender, high-risk disease status, and other key stratification, PFS in zanubrutinib group were superior to the BR group. In the ELEVATE R/R study[16], either acalabrutinib or ibrutinib was randomly assigned to 533 patients who were previously treated high-risk CLL—del (17p) or del (11q). The IRC-assessed ORR was 81.0% (95% CI, 75.8–85.2) for acalabrutinib and 77.0% (95% CI, 71.5–81.6). The median PFS of acalabrutinib (38.4 months in both groups) was non-inferior to ibrutinib. However, compared with ibrutinib, the incidence of atrial fibrillation/flutter (9.4% vs 16%; P = 0.02), hypertension (9.4% vs 23.2%), and bleeding events (38% vs 51.3%) were lower. There are differences in the discontinuation rates caused by AE, with acalabrutinib being 14.7% and ibrutinib being 21.3%. Zanubrutinib or ibrutinib was randomly assigned to 652 patients who had previously received CLL treatment in the ALPINE study[25]. Compared with ibrutinib, zanubrutinib treatment can improve the overall response (86.2% vs 75.7%, P < 0.01) and the 24-month PFS incidence (78.4% vs 65.9%, P = 0.002). Zanubrutinib was associated with a lower cumulative incidence of atrial fibrillation/flutter (5.2% vs 13.3%), but the incidence rate of neutropenia increased (29.3% vs 24.4%), while the infection rate did not increase (71.3% vs 73.1%). Compared to ibrutinib, events leading to discontinuation of medication with zanubrutinib are less common (14.5% vs 22.2%). Based on the results of these studies, zanubrutinib and acalabrutinib are preferred over ibrutinib due to their favorable safety profile, and zanubrutinib has superior efficacy compared with ibrutinib.
Our meta-analysis showed that the pooled ORR and CR rate of new-generation BTKi-based treatment for CLL were 92% and 10%, respectively, confirming their good efficacy. However, the I2 values for ORR and CR rate were 80.68% and 88.73%, which was quite heterogeneous. Meanwhile, the reason why the heterogeneity of subgroup analysis results did not decrease may be due to significant differences in sample size and individual heterogeneity in each study. Among all studies, six had a sample size of less than 30, while one study involved 327 patients.
According to the results of the analysis, the following new-generation BTKi-based therapy conditions yielded higher efficacy: < 65 years old, TN-CLL, and BTKi combination therapy. Sub-group analysis showed that the ORR and CR rates from acalabrutinib monotherapy for CLL were 87% and 3%, respectively, while zanubrutinib monotherapy showed OR and CR rates of 93% and 13%, respectively. The ORR and CR rates of acalabrutinib combined with chemotherapy were 97% and 22%. These results indicated that adding acalabrutinib to chemotherapy may improve efficacy. The ORR and CR rates of BTKi combination therapy were higher than those of BTKi monotherapy, suggesting that the efficacy of BTKi combination therapy was superior to BTKi monotherapy. Furthermore, zanubrutinib monotherapy yielded higher efficacy than acalabrutinib monotherapy, indicating that zanubrutinib may be the first choice in monotherapy for CLL compared to acalabrutinib. The head-to-head RCTs are still needed to compare the efficacy between zanubrutinib monotherapy and acalabrutinib monotherapy. Furthermore, we explored the impact of IGHV status on the efficacy of new-generation BTKi treatment. The results showed that there was no statistically significant difference in ORR between unmutated IGHV and mutated IGHV.
Ibrutinib had shown impressive survival data in CLL. In this study, the 24-month OS and PFS rates of patients who received the new-generation BTKi-based regimen were 94% and 86%, respectively. According to the subgroup analysis, the rates of BTKi combination therapy were higher than BTKi monotherapy (24-month OS: 96% vs 93%; 24-month PFS: 94% vs 85%). Zanubrutinib monotherapy was slightly better than acalabrutinib monotherapy (24-month OS: 95% vs 92%; 24-month PFS: 86% vs 83%). However, most studies did not reach the median OS, and pooling long-term survival outcomes was impossible due to the limited follow-up duration. The effects of new-generation BTKi on survival need to be further evaluated by extending the follow-up time. In our meta-analysis of ≥ grade 3 AEs, we found that hematology AEs were the most common AEs, with an incidence of 5–14%. The main manifestations as cytopenia, including neutropenia, anemia, and thrombocytopenia. The highest incidence of non-hematological AEs was hypertension, which was 4%. Other non-hematological AEs including diarrhea, fatigue, and upper respiratory tract infection were all 1%. Previous studies had shown that ibrutinib caused a high incidence of atrial fibrillation. This study’s pooled grade ≥ 3 atrial fibrillation rate was only 1%. The results of subgroup analysis indicated that BTKi combination therapy was associated with higher or similar rates of most AEs compared to BTKi monotherapy, which to some extent limited the utilization of BTKi combination therapy. The use of acalabrutinib monotherapy was associated with lower rates of neutropenia and hypertension and higher rates of thrombocytopenia and anemia compared to zanubrutinib monotherapy. No numerical differences in grade ≥ 3 diarrhea, fatigue, or upper respiratory tract infection were found between both acalabrutinib and zanubrutinib monotherapy. Differences in the safety profile between acalabrutinib and zanubrutinib can assist clinicians in selecting a specific BTKi based on patients’ comorbidities and/or preferences.
This meta-analysis has several limitations. (1) Our analysis has certain limitations due to the nature of the included studies, which are mostly prospective phase I/II clinical trials. The single-arm trials make difficult robust comparisons with other treatment options. (2) In our analysis, we identified significant heterogeneity, which could be attributed to the small sample size in some included studies and the infrequent incidence of certain events. (3) We included studies on acalabrutinib and zanubrutinib which accounted for the majority of the interventions, while tirabrutinib was only included in one. No research on orelabrutinib has been retrieved. The efficacy and safety of tirabrutinib and orelabrutinib cannot be well evaluated. (4) The survival data of most studies are incomplete. Most studies did not reach the median OS and PFS. Therefore, we only analyzed the 24-month OS and PFS from several studies. (6) Among all the retrieved studies, only acalabrutinib was used in combination therapy, while zanubrutinib and tirabrutinib were both used as monotherapy. (7) Since most studies have not separately shown the efficacy of gene mutations in patients with CLL, gene-related subgroup analysis has not been all carried out.
Conclusions
Overall, this meta-analysis has confirmed the excellent efficacy and safety of new-generation BTKi for CLL. The efficacy of BTKi combination therapy is superior to BTKi monotherapy, but its incidence of AEs is higher than monotherapy. The increased occurrence of adverse effects is attributed to the combination of multiple drugs, which raises the risk of drug interactions and side effects. Therefore, exploring safer combination treatment strategies is expected to become one of the future research priorities. Among the BTKi monotherapy, we mainly compare acalabrutinib and zanubrutinib. Zanubrutinib may be the preferred choice in monotherapy for CLL compared to acalabrutinib; both acalabrutinib and zanubrutinib have their advantages and disadvantages in terms of AEs, but the incidence of atrial fibrillation is low for both. Toxicity should be monitored by clinicians, and timely prevention and intervention should be provided as well. To verify our findings and establish the impact of new-generation BTKi on CLL, it is crucial to conduct large-scale multicenter studies and RCTs. Additionally, further studies are needed to determine the optimal schedule of BTKi for CLL treatment.
The following supporting information can be downloaded at: https://doi.org/10.5281/zenodo.7970370, Table S1: Search algorithm; Figure S1: Quality assessment of included randomized studies; Figure S2: Forest plots assessing the effect of age (≥ 65 vs < 65) on (A) ORR; (B) CRR; Figure S3: Forest plots assessing the effect of disease status ( TN-CLL vs RR-CLL) on (A) ORR; (B) CRR; Figure S4:Forest plots assessing the effect of treatment strategy (BTKi monotherapy vs BTKi combination therapy) on (A) ORR; (B) CRR; Figure S5: Forest plots assessing the effect of treatment strategy (acalabrutinib monotherapy vs. zanubrutinib monotherapy) on (A) ORR; (B) CRR; Figure S6: Forest plots assessing the effect of treatment strategy (acalabrutinib-based regimen) on (A) ORR; (B) CRR; Figure S7: Forest plots assessing the 24-months OS (A) BTKi; (B) acalabrutinib monotherapy; (C) zanubrutinib monotherapy; Figure S8: Forest plots assessing the 24-months PFS (A) BTKi; (B) acalabrutinib monotherapy; (C) zanubrutinib monotherapy; Figure S9: Forest plots assessing the effect of BTKi combination therapy vs BTKi monotherapy (A) 24-months OS; (B) 24-months PFS; Figure S10: Forest plots assessing the effect of disease status (TN-CLL vs RR-CLL) (A) 24-months OS; (B) 24-months PFS; Figure S11: Forest plot of the ORR for treatment with the unmutated IGHV vs. mutated IGHV (fixed effect model). RR is the effect size; Figure S12: Forest plots for pooled grade ≥ 3 (A) neutropenia; (B) anemia; (C) thrombocytopenia; (D) diarrhea; (E) fatigue; (F) upper respiratory tract infection; (G) atrial fibrillation; (H) hypertension.
Data availability
Data were extracted and analyzed from published articles available and accessible in the shared database. All datasets generated during the study are available upon reasonable request from the corresponding authors.
References
Hematology Committee of Chinese Medical Association (2022) The guidelines for diagnosis and treatment of chronic lymphocytic leukemia/small lymphocytic lymphoma in China (2022)[J]. Zhonghua xue ye xue za zhi= Zhonghua xueyexue zazhi 43(5):353–358
Siegel RL, Miller KD, Wagle NS et al (2023) Cancer statistics, 2023[J]. CA Cancer J Clin 73(1):17–48
Smith A, Howell D, Patmore R et al (2011) Incidence of haematological malignancy by sub-type: a report from the Haematological Malignancy Research Network[J]. Br J Cancer 105(11):1684–1692
Raedler L A. Imbruvica (ibrutinib), first-in-class Bruton's tyrosine kinase inhibitor, receives expanded indications for patients with relapsed chronic lymphocytic leukemia[J]. American health & drug benefits, 2015, 8(Spec Feature): 66. https://www.AHDBonline.com
Byrd JC, Furman RR, Coutre SE et al (2013) Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia[J]. N Engl J Med 369(1):32–42
Munir T, Brown JR, O’Brien S et al (2019) Final analysis from RESONATE: up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma[J]. Am J Hematol 94(12):1353–1363
Wen T, Wang J, Shi Y et al (2021) Inhibitors targeting Bruton’s tyrosine kinase in cancers: drug development advances[J]. Leukemia 35(2):312–332
Hallek M. International Workshop on Chronic Lymphocytic Leukemia. Guidelines for the diagnosis and treatement of chronic lymphocytic leukemia: a report from the Iternational Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group (1996) guidelines[J]. Blood 2008(111):5446–5456
Cheson BD, Fisher RI, Barrington SF et al (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification[J]. J Clin Oncol 32(27):3059
Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ et al (2019) Cochrane handbook for systematic reviews of interventions. Wiley, Chichester
Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J (2003) Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 73(9):712–716. https://doi.org/10.1046/j.1445-2197.2003.02748.x
Ghia P, Pluta A, Wach M et al (2022) Acalabrutinib versus investigator’s choice in relapsed/refractory chronic lymphocytic leukemia: final ASCEND trial results[J]. HemaSphere 6(12):e801
Byrd JC, Woyach JA, Furman RR et al (2021) Acalabrutinib in treatment-naive chronic lymphocytic leukemia[J]. Blood 137(24):3327–3338
Awan FT, Schuh A, Brown JR et al (2019) Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib[J]. Blood Adv 3(9):1553–1562
Woyach JA, Blachly JS, Rogers KA et al (2020) Acalabrutinib plus obinutuzumab in treatment-naïve and relapsed/refractory chronic lymphocytic leukemia[J]. Cancer Discov 10(3):394–405
Byrd JC, Hillmen P, Ghia P et al (2021) Acalabrutinib versus ibrutinib in previously treated chronic lymphocytic leukemia: results of the first randomized phase III trial[J]. J Clin Oncol 39(31):3441–3452
Sharman JP, Egyed M, Jurczak W et al (2020) Acalabrutinib with or without obinutuzumab versus chlorambucil and obinutuzumab for treatment-naive chronic lymphocytic leukaemia (ELEVATE-TN): a randomised, controlled, phase 3 trial[J]. The Lancet 395(10232):1278–1291
Davids MS, Lampson BL, Tyekucheva S et al (2021) Acalabrutinib, venetoclax, and obinutuzumab as a frontline treatment for chronic lymphocytic leukaemia: a single-arm, open-label, phase 2 study[J]. Lancet Oncol 22(10):1391–1402
Rogers KA, Thompson PA, Allan JN et al (2021) Phase II study of acalabrutinib in ibrutinibintolerant patients with relapsed/refractory chronic lymphocytic leukemia[J]. Haematologica 106(9):2364
Sun C, Nierman P, Kendall EK et al (2020) Clinical and biological implications of target occupancy in CLL treated with the BTK inhibitor acalabrutinib[J]. Blood 136(1):93–105
Soumerai JD, Mato AR, Dogan A et al (2021) Zanubrutinib, obinutuzumab, and venetoclax with minimal residual disease-driven discontinuation in previously untreated patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: a multicentre, single-arm, phase 2 trial[J]. The Lancet Haematology 8(12):e879–e890
Xu W, Yang S, Zhou K et al (2020) Treatment of relapsed/refractory chronic lymphocytic leukemia/small lymphocytic lymphoma with the BTK inhibitor zanubrutinib: phase 2, single-arm, multicenter study[J]. J Hematol Oncol 13:1–12
Tam CS, Brown JR, Kahl BS et al (2022) Zanubrutinib versus bendamustine and rituximab in untreated chronic lymphocytic leukaemia and small lymphocytic lymphoma (SEQUOIA): a randomised, controlled, phase 3 trial[J]. Lancet Oncol 23(8):1031–1043
Cull G, Burger JA, Opat S et al (2022) Zanubrutinib for treatment-naïve and relapsed/refractory chronic lymphocytic leukaemia: long-term follow-up of the phase I/II AU-003 study[J]. Br J Haematol 196(5):1209–1218
Brown JR, Eichhorst B, Hillmen P et al (2023) Zanubrutinib or ibrutinib in relapsed or refractory chronic lymphocytic leukemia[J]. N Engl J Med 388(4):319–332
Danilov AV, Herbaux C, Walter HS et al (2020) Phase Ib study of tirabrutinib in combination with idelalisib or entospletinib in previously treated chronic lymphocytic leukemia [J]. Clin Cancer Res 26(12):2810–2818
Alaggio R, Amador C, Anagnostopoulos I et al (2022) The 5th edition of the World Health Organization classification of haematolymphoid tumours: lymphoid neoplasms[J]. Leukemia 36(7):1720–1748
Byrd JC, Brown JR, O’Brien S et al (2014) Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia[J]. N Engl J Med 371(3):213–223
Burger JA, Tedeschi A, Barr PM et al (2015) Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia[J]. N Engl J Med 373(25):2425–2437
Shanafelt TD, Wang XV, Kay NE et al (2019) Ibrutinib–rituximab or chemoimmunotherapy for chronic lymphocytic leukemia[J]. N Engl J Med 381(5):432–443
Allan JN, Shanafelt T, Wiestner A et al (2022) Long-term efficacy of first-line ibrutinib treatment for chronic lymphocytic leukaemia in patients with TP53 aberrations: a pooled analysis from four clinical trials[J]. Br J Haematol 196(4):947–953
Barr PM, Owen C, Robak T et al (2022) Up to 8-year follow-up from RESONATE-2: first-line ibrutinib treatment for patients with chronic lymphocytic leukemia[J]. Blood Adv 6(11):3440–3450
US Food and Drug Administration (2022) Imbruvica (ibrutinib). Prescribing information[J]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/205552s035,215063s011lbl.pdf
NCCN Guidelines Version 1.2023 Chronic lymphocytic leukemia/small lymphocytic lymphoma.
Funding
This research was supported by the National Key R&D Program of China (No. 2021YFF0901404).
Author information
Authors and Affiliations
Contributions
Conceptualization, S.Y.; methodology, S.Y., W.Z., and X.Z.; software, S.Y. and X.Z.; validation, S.Y., X.Z., and F.C.; formal analysis, S.Y. and X.Z.; investigation, S.Y., X.Z., and F.C.; resources, S.Y. and X.Z.; data curation, S.Y., X.Z., and F.C.; writing—original draft preparation, S.Y. and X.Z.; writing—review and editing, S.Y., H.Z., W.L., and F.C.; visualization, S.Y. and R.Z.; supervision, F.C. and W.L.; project administration, W.L. and F.C.; funding acquisition, W.L. and F.C. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yin, S., Zheng, X., Zhang, W. et al. Efficacy and safety of new-generation Bruton tyrosine kinase inhibitors in chronic lymphocytic leukemia/small lymphocytic lymphoma: a systematic review and meta-analysis. Ann Hematol 103, 2231–2244 (2024). https://doi.org/10.1007/s00277-023-05486-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-023-05486-x