Abstract
Background
Postoperative adjuvant chemotherapy with S-1 for 1 year (corresponding to eight courses) is the standard treatment for pathological stage II gastric cancer. The phase III trial (JCOG1104) investigating the non-inferiority of four courses of S-1 to eight courses was terminated due to futility at the first interim analysis. To confirm the primary results, we reported the results after a 5-years follow-up in JCOG1104.
Methods
Patients histologically diagnosed with stage II gastric cancer after radical gastrectomy were randomly assigned to receive S-1 for eight or four courses. In detail, 80 mg/m2/day S-1 was administered for 4 weeks followed by a 2-week rest as a single course.
Results
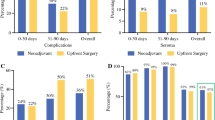
Between February 16, 2012, and March 19, 2017, 590 patients were enrolled and randomly assigned to 8-course (295 patients) and 4-course (295 patients) regimens. After a 5-years follow-up, the relapse-free survival at 3 years was 92.2% for the 8-course arm and 90.1% for the 4-course arm, and that at 5 years was 87.7% for the 8-course arm and 85.6% for the 4-course arm (hazard ratio 1.265, 95% CI 0.846–1.892). The overall survival at 3 years was 94.9% for the 8-course arm, 93.2% for the 4-course arm, and that at 5 years was 89.7% for the 8-course arm, and 88.6% for the 4-course arm (HR 1.121, 95% CI 0.719–1.749).
Conclusions
The survival of the four-course arm was slightly but consistently inferior to that of the eight-course arm. Eight-course S-1 should thus remain the standard adjuvant chemotherapy for pathological stage II gastric cancer.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T, Ohashi Y. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–93.
Japanese gastric cancer a: Japanese gastric cancer treatment guidelines 2021 (6th edition). Gastric Cancer 2023;26(1):1–25.
Yoshikawa T, Terashima M, Mizusawa J, Nunobe S, Nishida Y, Yamada T, Kaji M, Fukushima N, Hato S, Choda Y, et al. Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(3):208–16.
Japanese gastric cancer a: Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 2011;14(2):101–12.
Lan KKG, DeMets D. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659.
Fleming TR, Sharples K, McCall J, Moore A, Rodgers A, Stewart R. Maintaining confidentiality of interim data to enhance trial integrity and credibility. Clin Trials. 2008;5(2):157–67.
Kakeji Y, Ishikawa T, Suzuki S, Akazawa K, Irino T, Miyashiro I, Ono H, Suzuki H, Tanabe S, Kadowaki S, et al. A retrospective 5-year survival analysis of surgically resected gastric cancer cases from the Japanese Gastric Cancer Association nationwide registry (2001–2013). Gastric Cancer. 2022;25(6):1082–93.
Acknowledgements
We thank Megumi Miyazawa, Kumiko Yanagida, Harumi Kaba for data management. The study was supported in part by the National Cancer Center Research and Development Funds (23-A-16, 23-A-19, 26-A-4, 29-A-3, 2020-J-3, 2023-J-03) and by AMED under Grant Number JP17ck0106322. Participating Institutions (59 institutions) are Hakodate Goryoukaku Hospital, Keiyukai Sapporo Hospital, Iwate Medical University, National Hospital Organization, Sendai Medical Center, Miyagi Cancer Center, Yamagata Prefectural Central Hospital, Tochigi Cancer Center, National Defense Medical College, Saitama Cancer Center, Saitama Medical University International Medical Center, National Cancer Center Hospital East, Chiba Cancer Center, National Cancer Center Hospital, Tokyo Metropolitan Cancer and Infectious diseases Center Komagome Hospital, Tokyo Medical and Dental University Hospital, Cancer Institute Hospital of Japanese Foundation for Cancer Research, Toranomon Hospital, Tokyo Metropolitan Bokutoh Hospital, Kanagawa Cancer Center, Kitasato University School of Medicine, Yokohama City University Medical Center, Niigata Cancer Center Hospital, Nagaoka Chuo General Hospital, Toyama Prefectural Central Hospital, Ishikawa Prefectural Central Hospital, Gifu University School of Medicine, Gifu Municipal Hospital, Shizuoka General Hospital, Shizuoka Cancer Center, Aichi Cancer Center Hospital, Nagoya University School of Medicine, National Hospital Organization Kyoto Medical Center, Kyoto Second Red Cross Hospital, Osaka University Graduate School of Medicine, Kindai University Faculty of Medicine, Osaka International Cancer Institute, Osaka National Hospital, Osaka General Medical Center, Osaka Medical College, Toyonaka Municipal Hospital, Sakai City Medical Center, Kansai Medical University Hospital, Osaka Rosai Hospital, Kobe University Graduate School of Medicine, Kansai Rosai Hospital, Hyogo College of Medicine, Hyogo Cancer Center, Itami City Hospital, Tenri Hospital, Wakayama Medical University, School of Medicine, Shimane University Faculty of Medicine, Okayama University Hospital, Hiroshima University Hospital, Hiroshima City Hospital, Hiroshima City Asa Hospital, Fukuyama City Hospital, Tokushima Red Cross Hospital, National Hospital Organization Shikoku Cancer Center, Oita University Faculty of Medicine. Please see the appendix including each site from which patients were recruited, the name of principal investigator responsible for this site, and the number of patients which were recruited from that particular site.
Funding
The study was supported by the Japan Agency for Medical Research and Development, the Ministry of Health, Labour and Welfare of Japan, and the National Cancer Center Research and Development Fund.
Author information
Authors and Affiliations
Contributions
TYo, MS, and TS had the original idea for the study, and MS followed by MTer chaired the study group. TYo wrote the protocol, assisted by MTer, JM, HK, HF, TS, and MS. Statistical analyses were performed by JM. All the authors except JM, HK, and HF recruited patients into the study. JM, HK, and HF were responsible for data management, statistical analysis, and interpretation. TYo drafted the paper. MTer, JM, HK, HF, NB, TS, and MS revised the paper. All the authors reviewed the paper. All the authors approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
TYo had received grants from Japan Agency for Medical Research and Development (AMED) and The National Cancer Center Research and Development Fund during the conduct of the study and personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol, MSD, Daiichi Sankyo, Nippon Kayaku, Terumo, Otsuka, AstraZeneca, Pfizer, Daiichi Sankyo, Johnson and Johnson, Medtronic Japan, Intuitive Japan, and Olympus. MTer had received grants from Japan Agency for Medical Research and Development (AMED) and The National Cancer Center Research and Development Fund during the conduct of the study and personal fees from Taiho Pharmaceutical, Chugai Pharmaceutical, Ono Pharmaceutical, Bristol, MSD, Daiichi Sankyo, Nippon Kayaku, Eli Lilly, Pfizer, Daiichi Sankyo, Johnson and Johnson, Medtronic Japan, Intuitive Japan, and Olympus. JM had received grants from Ministry of Health, Labour and Welfare, Japan, grants from Japan Agency for Medical Research and Development (AMED) during the conduct of the study and personal fees from Taiho pharmaceutical and Chugai pharmaceutical; his spouse is an employee of Pfizer Japan Inc. SN had no conflict of interest. YN had no conflict of interest. TYma had received personal fee from Taiho and Johnson & Johnson. KM had no conflict of interest. TN had no conflict of interest. SH had no conflict of interest. YC had received personal fee from ONO and Bristol-Myers Squibb. HY had had no conflict of interest. KY had received personal fee from Abbott. Asahi Kasei Pharma, Chugai Pharma, Covidien, Daiichi Sankyo, Eisai, Eli Lilly, Johnson & Johnson, Kaken Pharm, Kyowa Kirin, Nippon Kayaku, Otsuka Pharm, Sanofi, Taiho, Takeda, TERUMO, Tsumura, and Yakult Honsya. KM had no conflict of interest. TM had no conflict of interest. MTsu had no conflict of interest. YKaw had no conflict of interest. HK had received grants from Ministry of Health, Labour and Welfare, Japan and from Japan Agency for Medical Research and Development (AMED). HF had received personal fee from Kyowa Kirin, Chuigai Parm and CMIC. YKur had received personal fee from Taiho, NB had received personal fee from Taiho, Ono, Bristol-Myers Squibb, Daiichi Sankyo, and Eli Lilly. TS had received personal fee from Taiho and Chugai. MS had received personal fee from Taiho, Chugai, and Yakult.
Human rights statement and informed consent
All the procedures were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the 1964 Declaration of Helsinki and later versions. Informed consent for inclusion in the study was obtained from all the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10120_2023_1447_MOESM1_ESM.pdf
Supplementary Fig. 1: A Analysis using the unaffected population (RFS, n = 513). B Analysis using the unaffected population (OS, n = 513); Supplementary Fig. 2: Cumulative incidence curve of recurrence (PDF 12360 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yoshikawa, T., Terashima, M., Mizusawa, J. et al. 5-year follow-up results of a JCOG1104 (OPAS-1) phase III non-inferiority trial to compare 4 courses and 8 courses of S-1 adjuvant chemotherapy for pathological stage II gastric cancer. Gastric Cancer 27, 155–163 (2024). https://doi.org/10.1007/s10120-023-01447-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-023-01447-5