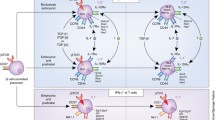
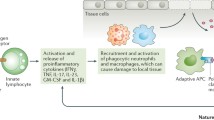
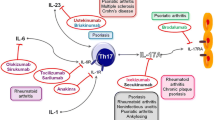
Abstract
It is now well established that Th17 lymphocytes associate with myriad immune-mediated inflammatory diseases. Over the past one and a half decades, a subset of Th17 lymphocytes viz. Th17.1 lymphocytes has been identified in pre-clinical and clinical models of inflammatory rheumatic diseases. These lymphocytes secrete IL-17A (signature cytokine of Th17 lymphocytes) as well as IFN-γ (the signature cytokine of Th1 lymphocytes). They express the chemokine markers for Th1 (CXCR3) as well as Th17 (CCR6) lymphocytes. Th17.1 lymphocytes also express the drug efflux protein p-glycoprotein, which associates with resistance to corticosteroids and other immunosuppressive drugs. This narrative review overviews the evidence regarding Th17.1 lymphocytes in different inflammatory rheumatic diseases. It is now recognized that Th17.1 lymphocytes are increased in the synovial fluid of affected joints in rheumatoid arthritis (RA) and associate with poor treatment response to abatacept. Th17.1 lymphocytes from synovial fluid of RA are less responsive to immunosuppression than those from the peripheral blood. In sarcoidosis, Th17.1 lymphocytes are concentrated in mediastinal lymph nodes and alveolar lining. Such Th17.1 lymphocytes in sarcoidosis are the predominant source of IFN-γ in the sarcoid lung. Th17.1 lymphocytes are elevated in lupus and Takayasu arteritis and associate with disease activity. Future studies should evaluate isolated Th17.1 lymphocytes from peripheral blood or sites of pathology such as synovial fluid and assess their modulation with immunosuppressive therapy in vitro. The analysis of gene expression signature of isolated Th17.1 lymphocytes might enable the identification of newer therapeutic strategies specifically targeting these cell populations in inflammatory rheumatic diseases.
Key Points |
• Th17.1 lymphocytes are a subset of Th17 lymphocytes secreting both IFN-γ and IL-17 • Th17.1 lymphocytes drive neutrophilic inflammation, granuloma formation, and corticosteroid resistance • Th17.1 lymphocytes are elevated in rheumatoid arthritis and sarcoidosis at sites of inflammation • Increased circulating Th17.1 lymphocytes have been identified in lupus and Takayasu arteritis and associate with active disease |



Similar content being viewed by others
Data availability
Data pertaining to this narrative review shall be shared on reasonable request to the corresponding author (Durga Prasanna Misra, durgapmisra@gmail.com).
Abbreviations
- αCD3:
-
Anti-CD3
- αCD28:
-
Anti-CD28
- AAV:
-
ANCA-associated vasculitis
- ACPA:
-
Anti-citrullinated peptide antibody
- ANCA:
-
Anti-neutrophil cytoplasmic antibody
- anti-dsDNA:
-
Antibodies to double-stranded deoxy ribonucleic acid
- BAL:
-
Bronchoalveolar lavage
- DAS28-CRP:
-
Disease activity score assessed using 28 joint count and C-reactive protein
- EGPA:
-
Eosinophilic granulomatosis with polyangiitis
- FACS:
-
Fluorescence-associated cell sorting
- GCA:
-
Giant cell arteritis
- GM-CSF:
-
Granulocyte monocyte colony-stimulating factor
- GPA:
-
Granulomatosis with polyangiitis
- IFN-γ:
-
Interferon gamma
- IL:
-
Interleukin
- JIA:
-
Juvenile idiopathic arthritis
- MACS:
-
Magnetic-associated cell sorting
- MDR 1:
-
Multidrug resistance protein 1
- mRNA:
-
Messenger ribonucleic acid
- PD-1:
-
Programmed cell death 1
- p-gp:
-
P-Glycoprotein
- PMA:
-
Phorbol myristate acetate
- PsA:
-
Psoriatic arthritis
- RA:
-
Rheumatoid arthritis
- scRNAseq:
-
Single-cell RNA sequencing
- SLE:
-
Systemic lupus erythematosus
- SLEDAI:
-
SLE disease activity index
- SS:
-
Sjogren’s syndrome
- TAK:
-
Takayasu arteritis
- Tc:
-
T cytotoxic lymphocytes
- TGF-β1:
-
Transforming growth factor beta 1
- Th:
-
T helper lymphocytes
- Treg:
-
Regulatory T lymphocytes
- VitD3:
-
1,25 Di-hydroxy vitamin D3
References
Mony JT, Khorooshi R, Owens T (2014) Chemokine receptor expression by inflammatory T cells in EAE. Front Cell Neurosci. 8. https://doi.org/10.3389/fncel.2014.00187
Watanabe S, Yamada Y, Murakami H (2020) Expression of Th1/Th2 cell-related chemokine receptors on CD4(+) lymphocytes under physiological conditions. Int J Lab Hematol 42:68–76. https://doi.org/10.1111/ijlh.13141
Yamaguchi H, Hiroi M, Mori K, et al. (2021) Simultaneous expression of Th1- and Treg-associated chemokine genes and CD4+, CD8+, and Foxp3+ cells in the premalignant lesions of 4NQO-induced mouse tongue tumorigenesis. Cancers 13. https://doi.org/10.3390/cancers13081835
Yang P, Qian FY, Zhang MF et al (2019) Th17 cell pathogenicity and plasticity in rheumatoid arthritis. J Leukoc Biol 106:1233–1240. https://doi.org/10.1002/jlb.4ru0619-197r
Tristão FSM, Rocha FA, Carlos D et al (2017) Th17-inducing cytokines IL-6 and IL-23 are crucial for granuloma formation during experimental paracoccidioidomycosis. Front Immunol 8:949. https://doi.org/10.3389/fimmu.2017.00949
Shen H, Chen ZW (2018) The crucial roles of Th17-related cytokines/signal pathways in M. tuberculosis infection. Cell Mol Immunol 15:216–225. https://doi.org/10.1038/cmi.2017.128
Bordon Y (2014) Spotting the troublemakers. Nat Rev Immunol 14:64–65. https://doi.org/10.1038/nri3610
Ramesh R, Kozhaya L, McKevitt K et al (2014) Pro-inflammatory human Th17 cells selectively express P-glycoprotein and are refractory to glucocorticoids. J Exp Med 211:89–104. https://doi.org/10.1084/jem.20130301
Acosta-Rodriguez EV, Rivino L, Geginat J et al (2007) Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 8:639–646. https://doi.org/10.1038/ni1467
Celada LJ, Kropski JA, Herazo-Maya JD, et al. (2018) PD-1 up-regulation on CD4(+) T cells promotes pulmonary fibrosis through STAT3-mediated IL-17A and TGF-beta1 production. Sci Transl Med 10. https://doi.org/10.1126/scitranslmed.aar8356
Singh K, Rathore U, Rai MK et al (2022) Novel Th17 lymphocyte populations, Th17.1 and PD1+Th17, are increased in Takayasu arteritis, and both Th17 and Th17.1 sub-populations associate with active disease. J Inflamm Res 15:1521–1541. https://doi.org/10.2147/JIR.S355881
Benham H, Norris P, Goodall J et al (2013) Th17 and Th22 cells in psoriatic arthritis and psoriasis. Arthritis Res Ther 15:R136. https://doi.org/10.1186/ar4317
Raychaudhuri SK, Saxena A, Raychaudhuri SP (2015) Role of IL-17 in the pathogenesis of psoriatic arthritis and axial spondyloarthritis. Clin Rheumatol 34:1019–1023. https://doi.org/10.1007/s10067-015-2961-7
Taams LS (2020) Interleukin-17 in rheumatoid arthritis: trials and tribulations. J Exp Med 217:e20192048. https://doi.org/10.1084/jem.20192048
Nistala K, Moncrieffe H, Newton KR, Varsani H, Hunter P, Wedderburn LR (2008) Interleukin-17-producing T cells are enriched in the joints of children with arthritis, but have a reciprocal relationship to regulatory T cell numbers. Arthritis Rheum 58:875–887. https://doi.org/10.1002/art.23291
Clinical trial of secukinumab in juvenile psoriatic arthritis and enthesitis-related arthritis https://clinicaltrials.gov/ct2/show/results/NCT03031782 [Accessed on 09 March 2022].
Nistala K, Adams S, Cambrook H et al (2010) Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc Natl Acad Sci U S A 107:14751–14756. https://doi.org/10.1073/pnas.1003852107
Jimeno R, Gomariz RP, Garín M et al (2015) The pathogenic Th profile of human activated memory Th cells in early rheumatoid arthritis can be modulated by VIP. J Mol Med (Berl) 93:457–467. https://doi.org/10.1007/s00109-014-1232-4
Jimeno R, Leceta J, Garín M et al (2015) Th17 polarization of memory Th cells in early arthritis: the vasoactive intestinal peptide effect. J Leukoc Biol 98:257–269. https://doi.org/10.1189/jlb.3A0714-327R
Paulissen SM, van Hamburg JP, Davelaar N et al (2015) CCR6(+) Th cell populations distinguish ACPA positive from ACPA negative rheumatoid arthritis. Arthritis Res Ther 17:344. https://doi.org/10.1186/s13075-015-0800-5
Jeffery LE, Henley P, Marium N et al (2018) Decreased sensitivity to 1,25-dihydroxyvitamin D3 in T cells from the rheumatoid joint. J Autoimmun 88:50–60. https://doi.org/10.1016/j.jaut.2017.10.001
Dankers W, Davelaar N, van Hamburg JP, van de Peppel J, Colin EM, Lubberts E (2019) Human memory Th17 cell populations change into anti-inflammatory cells with regulatory capacity upon exposure to active vitamin D. Front Immunol 10:1504. https://doi.org/10.3389/fimmu.2019.01504
Dankers W, den Braanker H, Paulissen SMJ et al (2021) The heterogeneous human memory CCR6+ T helper-17 populations differ in T-bet and cytokine expression but all activate synovial fibroblasts in an IFNγ-independent manner. Arthritis Res Ther 23:157. https://doi.org/10.1186/s13075-021-02532-9
Maeda S, Osaga S, Maeda T et al (2019) Circulating Th17.1 cells as candidate for the prediction of therapeutic response to abatacept in patients with rheumatoid arthritis: an exploratory research. PLoS One 14:e0215192. https://doi.org/10.1371/journal.pone.0215192
Abimannan T, Peroumal D, Parida JR, Barik PK, Padhan P, Devadas S (2016) Oxidative stress modulates the cytokine response of differentiated Th17 and Th1 cells. Free Radic Biol Med 99:352–363. https://doi.org/10.1016/j.freeradbiomed.2016.08.026
Koga T, Sato T, Umeda M et al (2016) Successful treatment of palmoplantar pustulosis with rheumatoid arthritis, with tofacitinib: impact of this JAK inhibitor on T-cell differentiation. Clin Immunol 173:147–148. https://doi.org/10.1016/j.clim.2016.10.003
den Braanker H, Razawy W, Wervers K et al (2022) Characterizing memory T helper cells in patients with psoriasis, subclinical, or early psoriatic arthritis using a machine learning algorithm. Arthritis Res Ther 24:28. https://doi.org/10.1186/s13075-021-02714-5
Facco M, Cabrelle A, Teramo A et al (2011) Sarcoidosis is a Th1/Th17 multisystem disorder. Thorax 66:144. https://doi.org/10.1136/thx.2010.140319
Jain A, Singh H, Nath A et al (2020) Distinct T-cell immunophenotypic signature in a subset of sarcoidosis patients with arthritis. J R Coll Physicians Edinb 50:226–232. https://doi.org/10.4997/jrcpe.2020.304
Ramstein J, Broos CE, Simpson LJ et al (2016) IFN-γ-producing T-helper 17.1 cells are increased in sarcoidosis and are more prevalent than T-helper type 1 cells. Am J Respir Crit Care Med 193:1281–1291. https://doi.org/10.1164/rccm.201507-1499OC
Broos CE, Koth LL, van Nimwegen M et al (2018) Increased T-helper 17.1 cells in sarcoidosis mediastinal lymph nodes. Eur Respir J 51:1701124. https://doi.org/10.1183/13993003.01124-2017
Arger NK, Machiraju S, Allen IE, Woodruff PG, Koth LL (2020) T-bet expression in peripheral Th17.0 cells is associated with pulmonary function changes in sarcoidosis. Front Immunol 11:1129. https://doi.org/10.3389/fimmu.2020.01129
Lepzien R, Nie M, Czarnewski P et al (2022) Pulmonary and blood dendritic cells from sarcoidosis patients more potently induce IFNγ-producing Th1 cells compared with monocytes. J Leukoc Biol 111:857–866. https://doi.org/10.1002/jlb.5a0321-162r
Lomax AJ, McGuire HM, McNeil C et al (2017) Immunotherapy-induced sarcoidosis in patients with melanoma treated with PD-1 checkpoint inhibitors: case series and immunophenotypic analysis. Int J Rheum Dis 20:1277–1285. https://doi.org/10.1111/1756-185x.13076
Koth LL, Harmacek LD, White EK et al (2021) Defining CD4 T helper and T regulatory cell endotypes of progressive and remitting pulmonary sarcoidosis (BRITE): protocol for a US-based, multicentre, longitudinal observational bronchoscopy study. BMJ Open 11:e056841. https://doi.org/10.1136/bmjopen-2021-056841
Koga T, Ichinose K, Kawakami A, Tsokos GC (2019) The role of IL-17 in systemic lupus erythematosus and its potential as a therapeutic target. Expert Rev Clin Immunol 15:629–637. https://doi.org/10.1080/1744666x.2019.1593141
Zhong W, Jiang Y, Ma H, Wu J, Jiang Z, Zhao L (2017) Elevated levels of CCR6(+) T helper 22 cells correlate with skin and renal impairment in systemic lupus erythematosus. Sci Rep 7:12962. https://doi.org/10.1038/s41598-017-13344-w
Zickert A, Amoudruz P, Sundström Y, Rönnelid J, Malmström V, Gunnarsson I (2015) IL-17 and IL-23 in lupus nephritis - association to histopathology and response to treatment. BMC Immunol 16:7. https://doi.org/10.1186/s12865-015-0070-7
Shenoy S, Chaurasia S, Edavalath S et al (2018) Effect of induction therapy on circulating T-helper 17 and T-regulatory cells in active proliferative lupus nephritis. Int J Rheum Dis 21:1040–1048. https://doi.org/10.1111/1756-185x.13272
Jakiela B, Kosałka J, Plutecka H, Bazan-Socha S, Sanak M, Musiał J (2018) Facilitated expansion of Th17 cells in lupus nephritis patients. Clin Exp Immunol 194:283–294. https://doi.org/10.1111/cei.13196
Zhong W, Jiang Z, Wu J, Jiang Y, Zhao L (2018) CCR6(+) Th cell distribution differentiates systemic lupus erythematosus patients based on anti-dsDNA antibody status. PeerJ 6:e4294. https://doi.org/10.7717/peerj.4294
Psianou K, Panagoulias I, Papanastasiou AD et al (2018) Clinical and immunological parameters of Sjögren’s syndrome. Autoimmun Rev 17:1053–1064. https://doi.org/10.1016/j.autrev.2018.05.005
Peng X, Lu Y, Wei J et al (2021) A cohort study of T helper 17 cell-related cytokine levels in tear samples of systemic lupus erythematosus and Sjögren’s syndrome patients with dry eye disease. Clin Exp Rheumatol 39(Suppl 133):159–165
Li B, Xing Y, Gan Y, He J, Hua H (2021) Labial gland-derived mesenchymal stem cells and their exosomes ameliorate murine Sjögren’s syndrome by modulating the balance of Treg and Th17 cells. Stem Cell Res Ther 12:478. https://doi.org/10.1186/s13287-021-02541-0
Koga T, Mizokami A, Nakashima M et al (2016) Histological improvement in salivary gland along with effector memory Th17-1 cell reduction in a primary Sjogren’s syndrome patient with dermatomyositis and diffuse large B-cell lymphoma by R-CHOP therapy. Clin Immunol 165:35–37. https://doi.org/10.1016/j.clim.2016.03.005
Guo H, Ju Y, Choi M et al (2022) Supra-lacrimal protein-based carriers for cyclosporine A reduce Th17-mediated autoimmunity in murine model of Sjögren’s syndrome. Biomaterials 283:121441. https://doi.org/10.1016/j.biomaterials.2022.121441
Wilde B, Thewissen M, Damoiseaux J, van Paassen P, Witzke O, Tervaert JW (2010) T cells in ANCA-associated vasculitis: what can we learn from lesional versus circulating T cells? Arthritis Res Ther 12:204. https://doi.org/10.1186/ar2923
Deng J, Younge BR, Olshen RA, Goronzy JJ, Weyand CM (2010) Th17 and Th1 T-cell responses in giant cell arteritis. Circulation 121:906–915. https://doi.org/10.1161/circulationaha.109.872903
Saadoun D, Garrido M, Comarmond C et al (2015) Th1 and Th17 cytokines drive inflammation in Takayasu arteritis. Arthritis Rheumatol 67:1353–1360. https://doi.org/10.1002/art.39037
Misra DP, Chaurasia S, Misra R (2016) Increased circulating Th17 cells, serum IL-17A, and IL-23 in Takayasu arteritis. Autoimmune Dis 2016:7841718. https://doi.org/10.1155/2016/7841718
Drozdzik M, Rudas T, Pawlik A et al (2006) The effect of 3435C>T MDR1 gene polymorphism on rheumatoid arthritis treatment with disease-modifying antirheumatic drugs. Eur J Clin Pharmacol 62:933–937. https://doi.org/10.1007/s00228-006-0192-1
Takatori R, Takahashi KA, Tokunaga D et al (2006) ABCB1 C3435T polymorphism influences methotrexate sensitivity in rheumatoid arthritis patients. Clin Exp Rheumatol 24:546–554
Yang XY, Xu DH (2007) MDR1(ABCB1) gene polymorphisms associated with steroid-induced osteonecrosis of femoral head in systemic lupus erythematosus. Pharmazie 62:930–932
Gonzalez TP, Mucenic T, Brenol JC, Xavier RM, Schiengold M, Chies JA (2008) ABCB1 C1236T, G2677T/A and C3435T polymorphisms in systemic lupus erythematosus patients. Braz J Med Biol Res 41:769–772. https://doi.org/10.1590/s0100-879x2008000900005
Ranganathan P, Culverhouse R, Marsh S et al (2008) Methotrexate (MTX) pathway gene polymorphisms and their effects on MTX toxicity in Caucasian and African American patients with rheumatoid arthritis. J Rheumatol 35:572–579
Chen J, Chen L, Mao N, Liu Y (2012) Association of the MDR1 3435 polymorphism in patients with refractory rheumatoid arthritis in a Chinese population. Rheumatol Int 32:3127–3130. https://doi.org/10.1007/s00296-011-2088-3
Prasad S, Tripathi D, Rai MK, Aggarwal S, Mittal B, Agarwal V (2014) Multidrug resistance protein-1 expression, function and polymorphisms in patients with rheumatoid arthritis not responding to methotrexate. Int J Rheum Dis 17:878–886. https://doi.org/10.1111/1756-185x.12362
Jorgensen C, Sun R, Rossi JF et al (1995) Expression of a multidrug resistance gene in human rheumatoid synovium. Rheumatol Int 15:83–86. https://doi.org/10.1007/bf00262714
Yudoh K, Matsuno H, Nakazawa F, Yonezawa T, Kimura T (1999) Increased expression of multidrug resistance of P-glycoprotein on Th1 cells correlates with drug resistance in rheumatoid arthritis. Arthritis Rheum 42:2014–2015. https://doi.org/10.1002/1529-0131(199909)42:9%3c2014::AID-ANR32%3e3.0.CO;2-R
Tsujimura S, Saito K, Nakayamada S, Nakano K, Tanaka Y (2005) Clinical relevance of the expression of P-glycoprotein on peripheral blood lymphocytes to steroid resistance in patients with systemic lupus erythematosus. Arthritis Rheum 52:1676–1683. https://doi.org/10.1002/art.21032
Hider SL, Owen A, Hartkoorn R et al (2006) Down regulation of multidrug resistance protein-1 expression in patients with early rheumatoid arthritis exposed to methotrexate as a first disease-modifying antirheumatic drug. Ann Rheum Dis 65:1390–1393. https://doi.org/10.1136/ard.2005.049189
Henmi K, Yoshida M, Yoshikawa N, Hirano T (2008) P-glycoprotein functions in peripheral-blood CD4+ cells of patients with systemic lupus erythematosus. Biol Pharm Bull 31:873–878. https://doi.org/10.1248/bpb.31.873
Lu MC, Lai NS, Li KJ, Hsieh SC, Wu CH, Yu CL (2008) Increased multidrug resistance-associated protein activity in mononuclear cells of patients with systemic lupus erythematosus. Clin Exp Rheumatol 26:638–645
Tsujimura S, Saito K, Nawata M, Nakayamada S, Tanaka Y (2008) Overcoming drug resistance induced by P-glycoprotein on lymphocytes in patients with refractory rheumatoid arthritis. Ann Rheum Dis 67:380–388. https://doi.org/10.1136/ard.2007.070821
Agarwal V, Mittal SK, Misra R (2009) Expression of multidrug resistance-1 protein correlates with disease activity rather than the refractoriness to methotrexate therapy in rheumatoid arthritis. Clin Rheumatol 28:427–433. https://doi.org/10.1007/s10067-008-1071-1
Suzuki K, Saito K, Tsujimura S et al (2010) Tacrolimus, a calcineurin inhibitor, overcomes treatment unresponsiveness mediated by P-glycoprotein on lymphocytes in refractory rheumatoid arthritis. J Rheumatol 37:512–520. https://doi.org/10.3899/jrheum.090048
Zhang B, Shi Y, Lei TC (2012) Detection of active P-glycoprotein in systemic lupus erythematosus patients with poor disease control. Exp Ther Med 4:705–710. https://doi.org/10.3892/etm.2012.667
Ragab SM, Soliman MA (2013) P-glycoprotein-1 functional activity in CD5+CD7+ and CD20+ lymphocytes in systemic lupus erythematosus children: relation to disease activity, complications and steroid response. Egypt J Immunol 20:101–115
Kansal A, Tripathi D, Rai MK, Agarwal V (2016) Persistent expression and function of P-glycoprotein on peripheral blood lymphocytes identifies corticosteroid resistance in patients with systemic lupus erythematosus. Clin Rheumatol 35:341–349. https://doi.org/10.1007/s10067-015-3079-7
Perez-Guerrero EE, Gamez-Nava JI, Muñoz-Valle JF et al (2018) Serum levels of P-glycoprotein and persistence of disease activity despite treatment in patients with systemic lupus erythematosus. Clin Exp Med 18:109–117. https://doi.org/10.1007/s10238-017-0459-0
Diamanti AP, Rosado M, Germano V et al (2011) Reversion of resistance to immunosuppressive agents in three patients with psoriatic arthritis by cyclosporine A: modulation of P-glycoprotein function. Clin Immunol 138:9–13. https://doi.org/10.1016/j.clim.2010.10.001
Edavalath S, Rai MK, Gupta V et al (2022) Tacrolimus induces remission in refractory and relapsing lupus nephritis by decreasing P-glycoprotein expression and function on peripheral blood lymphocytes. Rheumatol Int. https://doi.org/10.1007/s00296-021-05057-1
Misra DP, Rathore U, Patro P, Agarwal V, Sharma A (2021) Disease-modifying anti-rheumatic drugs for the management of Takayasu arteritis—a systematic review and meta-analysis. Clin Rheumatol 40:4391–4416. https://doi.org/10.1007/s10067-021-05743-2
Kiner E, Willie E, Vijaykumar B et al (2021) Gut CD4(+) T cell phenotypes are a continuum molded by microbes, not by T(H) archetypes. Nat Immunol 22:216–228. https://doi.org/10.1038/s41590-020-00836-7
van Beek JJP, Rescigno M, Lugli E (2021) A fresh look at the T helper subset dogma. Nat Immunol 22:104–105. https://doi.org/10.1038/s41590-020-00858-1
Gao Y, Dunlap G, Elahee M, Rao DA (2021) Patterns of T-cell phenotypes in rheumatic diseases from single-cell studies of tissue. ACR Open Rheumatol 3:601–613. https://doi.org/10.1002/acr2.11296
Yazar S, Alquicira-Hernandez J, Wing K et al (2022) Single-cell eQTL mapping identifies cell type-specific genetic control of autoimmune disease. Science 376:eabf3041. https://doi.org/10.1126/science.abf3041
Acknowledgements
Durga Prasanna Misra acknowledges support from Indian Council of Medical Research (Grant No 5/4/1-2/2019-NCD-II) for his research on Takayasu arteritis.
Author information
Authors and Affiliations
Contributions
The conception and design of the study—DPM, VA; acquisition of data, analysis, and interpretation of data—DPM;
drafting the article—DPM; revising it critically for important intellectual content—VA; final approval of the version to be submitted—DPM, VA;
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved—DPM, VA.
Corresponding author
Ethics declarations
Disclosures
None.
Disclaimer
The funding agency had no role in the actual conduct or reporting of this review.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Misra, D.P., Agarwal, V. Th17.1 lymphocytes: emerging players in the orchestra of immune-mediated inflammatory diseases. Clin Rheumatol 41, 2297–2308 (2022). https://doi.org/10.1007/s10067-022-06202-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10067-022-06202-2