Abstract
Objective
The purpose in the study was to evaluate the effect of biogenic silver nanoparticles (Ag NPs) synthesized by the green synthesis method on dentin bond strength in three different universal adhesives and investigate their antibiofilm activity against Streptococcus mutans (S. mutans).
Materials and methods
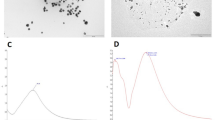
Three different universal adhesives (single bond universal, all-bond universal, and clearfil universal) were used in this study. Ag NPs were synthesized using rose hip (Rosa canina) extract as a reducing and stabilizing agent and they were characterized with STEM, UV–vis spectrophotometer, DLS, and zeta potential. Ag NPs were added to the adhesive resins at a rate of 0.05% (w/w), and their homogeneous distribution in the adhesive was determined using EDX spectrometry. Samples in all groups were tested at baseline-after 5000 and 10,000 thermal cycles. Adhesive composite discs were used for the live/dead analysis of S. mutans, MTT metabolic activity test, lactic acid production, and determination of colony-forming unit (CFU) values (n = 3). Ninety extracted caries-free human third molars were used to determine microtensile bond strength (μTBS) (n = 10). After the universal adhesive was applied to the dentin surface, composite resin (Z550 XT, 3 M ESPE, USA) was placed and sections were taken to form a composite-dentin stick of 1 mm × 1 mm wideness and 8-mm length. The sticks were broken at a rate of 1 mm/min under uniaxial tension in a universal testing machine, and the failure modes were determined by SEM. One-way analysis of variance (ANOVA) for antibacterial tests and two-way analysis of variance for μTBS tests were performed (p < 0.05).
Results
All universal adhesive groups containing Ag NPs showed higher antibacterial activity than control groups without Ag NPs (p < 0.05). There was a statistically significant difference in the live/dead assay analysis, MTT metabolic activity test, lactic acid production, and CFU values in the thermal cycled Ag NPs groups (p < 0.05). Antibacterial activity decreased in groups containing Ag NPs subjected to 10,000 thermal cycles. The highest lactic acid production 11.06 (± 0.629) and CFUs 7.61 (± 0.304), live bacteria 31.13 (± 0.466), and S. mutans MTT metabolic activity 0.29 (± 0.376) at AU (All-Bond Universal—Ag NPs) 10,000 thermal cycles group. There was no difference in μTBS values between the initial and 5000 thermal cycle groups, there was a difference between the 10,000 thermal cycle groups. The CU (Clearfil Universal—Ag NPs) group subjected to 10,000 thermal cycles showed lower μTBS 11.1 (± 3.2).
Conclusion
In conclusion, universal adhesives containing biogenic Ag NPs showed higher antibacterial activity than the control groups and did not reduce the μTBS.
Clinical relevance
Antibacterial universal adhesives can contribute to restoration success in clinical applications by reducing residual bacteria and preventing secondary caries formation.





Similar content being viewed by others
Data Availability
The journal has the right to use data regarding the article.
References
Twetman S (2018) Prevention of dental caries as a non-communicable disease. Eur J Oral Sci 126:19–25. https://doi.org/10.1111/eos.12528
Krzyściak W, Jurczak A, Kościelniak D, Bystrowska B, Skalniak A (2014) The virulence of Streptococcus mutans and the ability to form biofilms. Eur J Clin Microbiol Infect Dis 33:499–515. https://doi.org/10.1007/s10096-013-1993-7
Josic U, Mazzitelli C, Maravic T, Radovic I, Jacimovic J, Mancuso E, Florenzano F, Breschi L, Mazzoni A (2022) The influence of selective enamel etch and self-etch mode of universal adhesives’ application on clinical behavior of composite restorations placed on non-carious cervical lesions: a systematic review and meta-analysis. Dent Mater 38:472–488. https://doi.org/10.1016/j.dental.2022.01.002
Al Tuma RR, Yassir YA (2023) Effect of calcium fluoride nanoparticles in prevention of demineralization during orthodontic fixed appliance treatment: a randomized clinical trial. Eur J Orthod 31(45):122–132. https://doi.org/10.1093/ejo/cjac055
Ahn SJ, Lee SJ, Kook JK, Lim BS (2009) Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent Mater 25:206–213. https://doi.org/10.1016/j.dental.2008.06.002
Besinis A, De Peralta T, Handy RD (2014) The antibacterial effects of silver, titanium dioxide and silica dioxide nanoparticles compared to the dental disinfectant chlorhexidine on Streptococcus mutans using a suite of bioassays. Nanotoxicology 8:1–16. https://doi.org/10.3109/17435390.2012.742935
Schmalz G, Hickel R, van Landuyt KL, Reichl FX (2017) Nanoparticles in dentistry. Dent Mater 33:1298–1314. https://doi.org/10.1016/j.dental.2017.08.193
Ahmed O et al (2022) Plant extract-synthesized silver nanoparticles for application in dental therapy. Pharmaceutics 14:380. https://doi.org/10.3390/pharmaceutics14020380
Ekrikaya S, Yilmaz E, Celik C, Demirbuga S, Ildiz N, Demirbas A, Ocsoy I (2021) Investigation of ellagic acid rich-berry extracts directed silver nanoparticles synthesis and their antimicrobial properties with potential mechanisms towards Enterococcus faecalis and Candida albicans. J Biotechnol 341:155–162. https://doi.org/10.1016/j.jbiotec.2021.09.020
Cheng YJ, Zeiger DN, Howarter JA, Zhang X, Lin NJ, Antonucci JM, Lin-Gibson S (2011) In situ formation of silver nanoparticles in photocrosslinking polymers. J Biomed Mater Res B Appl Biomater 97(124):131. https://doi.org/10.1002/jbm.b.31793
Fan C, Chu L, Rawls HR, Norling BK, Cardenas HL, Whang K (2011) Development of an antimicrobial resin–a pilot study. Dent Mater 27:322–328. https://doi.org/10.1016/j.dental.2010.11.008
Arslan S, Ekrikaya S, Ildiz N, Yusufbeyoglu S, Ocsoy İ (2023) Evaluation of the antibacterial activity of dental adhesive containing biogenic silver nanoparticles decorated nanographene oxide nanocomposites (Ag@ nGO NCs) and effect on bond strength to dentine. Odontology, 1–14. https://doi.org/10.1007/s10266-023-00836-7
Demirbas A, Welt BA, Ocsoy I (2016) Biosynthesis of red cabbage extract directed Ag NPs and their effect on the loss of antioxidant activity. Mater Lett 179:20–23. https://doi.org/10.1016/j.matlet.2016.05.056
Zhang D, Ma XL, Gu Y, Huang H, Zhang GW (2020) Green synthesis of metallic nanoparticles and their potential applications to treat cancer. Front Chem 29(8):799. https://doi.org/10.3389/fchem.2020.00799
Wei H, Wang Z, Zhang J, House S, Gao YG, Yang L, Robinson H, Tan LH, Xing H, Hou C, Robertson IM, Zuo JM, Lu Y (2011) Time-dependent, protein-directed growth of gold nanoparticles within a single crystal of lysozyme. Nat Nanotechnol 6:93–97. https://doi.org/10.1038/nnano.2010.280
Some S et al (2019) Effect of feed supplementation with biosynthesized silver nanoparticles using leaf extract of Morus indica L. V1 on Bombyx mori L.(Lepidoptera: Bombycidae). Sci Rep 9:14839. https://doi.org/10.1038/s41598-019-50906-6
Salem SS, Fouda A (2021) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199:344–370. https://doi.org/10.1007/s12011-020-02138-3
Kaur N, Singh A, Ahmad W (2023) Microwave assisted green synthesis of silver nanoparticles and its application: a review. J Inorg Organomet Polym Mater 33:663–672
Demirbas A, Büyükbezirci K, Celik C, Kislakci E, Karaagac Z, Gokturk E, Kati A, Cimen B, Yilmaz V, Ocsoy I (2019) Synthesis of long-term stable gold nanoparticles benefiting from red raspberry (Rubus idaeus), strawberry (Fragaria ananassa), and blackberry (Rubus fruticosus) Extracts-gold ıon complexation and ınvestigation of reaction conditions. ACS Omega 4:18637–18644. https://doi.org/10.1021/acsomega.9b02469
Koca FD, Demirezen Yilmaz D, Ertas Onmaz N, Yilmaz E, Ocsoy I (2020) Green synthesis of allicin based hybrid nanoflowers with evaluation of their catalytic and antimicrobial activities. Biotechnol Lett 42:1683–1690. https://doi.org/10.1007/s10529-020-02877-2
Ocsoy I, Temiz M, Celik C, Altinsoy B, Yilmaz V, Duman F (2017) A green approach for formation of silver nanoparticles on magnetic graphene oxide and highly effective antimicrobial activity and reusability. J Mol Liq 227:147–152. https://doi.org/10.1016/j.molliq.2016.12.015
Sreenivasalu PKP, Dora CP, Swami R, Jasthi VC, Shiroorkar PN, Nagaraja S, Asdaq SMB, Anwer MK (2022) Nanomaterials in dentistry: current applications and future scope. Nanomaterials (Basel) 12:1676. https://doi.org/10.3390/nano12101676
Chrubasik C, Roufogalis BD, Müller-Ladner U, Chrubasik S (2008) A systematic review on the Rosa canina effect and efficacy profiles. Phytother Res 22:725–733. https://doi.org/10.1002/ptr.2400
Zhang K, Cheng L, Imazato S, Antonucci JM, Lin NJ, Lin-Gibson S, Bai Y, Xu HH (2013) Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J Dent 41:464–474. https://doi.org/10.1016/j.jdent.2013.02.001
Li F, Weir MD, Fouad AF, Xu HH (2014) Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent Mater 30:182–191. https://doi.org/10.1016/j.dental.2013.11.004
Li F, Weir MD, Chen J, Xu HH (2013) Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent Mater 29:450–461. https://doi.org/10.1016/j.dental.2013.01.012
Yang Y, Ding Y, Fan Y, Ren L, Tang X, Meng X (2021) Application of silver nanoparticles in situ synthesized in dental adhesive resin. Int J Adhes Adhes 108:02890. https://doi.org/10.1016/j.ijadhadh.2021.102890
Shafiei F, Memarpour M, Jowkar Z (2020) Effect of silver antibacterial agents on bond strength of fiber posts to root dentin. Braz Dent J Dental Journal 31:409–416. https://doi.org/10.1590/0103-6440202003300
Akram Z, Aati S, Clode P, Saunders M, Ngo H, Fawzy AS (2022) Formulation of nano-graphene doped with nano silver modified dentin bonding agents with enhanced interfacial stability and antibiofilm properties. Dent Mater 38:347–362. https://doi.org/10.1016/j.dental.2021.12.016
Prabhu S, Poulose EK (2012) Silver nanoparticles: mechanism of antimicrobial action, synthesis, medical applications, and toxicity effects. Int Nano Lett 2:1–10
Roy A, Bulut O, Some S, Mandal AK, Yilmaz MD (2019) Green synthesis of silver nanoparticles: biomolecule-nanoparticle organizations targeting antimicrobial activity. RSC Adv 9:2673–2702. https://doi.org/10.1039/c8ra08982e
Ghorbani HR, Safekordi AA, Attar H, Sorkhabadi SM (2011) Biological and non-biological methods for silver nanoparticles synthesis. Chem Biochem Eng Q 25:317–326
Palma PJ, Marques JA, Antunes M, Falacho RI, Sequeira D, Roseiro L, Santos JM, Ramos JC (2021) Effect of restorative timing on shear bond strength of composite resin/calcium silicate-based cements adhesive interfaces. Clin Oral Investig 25:3131–3139. https://doi.org/10.1007/s00784-020-03640-7
Hardan L, Bourgi R, Kharouf N, Mancino D, Zarow M, Jakubowicz N, Haikel Y, Cuevas-Suárez CE (2021) Bond strength of universal adhesives to dentin: a systematic review and meta-analysis. Polymers 13:814. https://doi.org/10.3390/polym13050814
Malacarne J, Carvalho RM, de Goes MF, Svizero N, Pashley DH, Tay FR, de Oliveira Carrilho MR, Cynthia KY, Carrilho MR (2006) Water sorption/solubility of dental adhesive resins. Dent Mater 22:973–980. https://doi.org/10.1016/j.dental.2005.11.020
Moule CA, Angelis F, Kim GH, Le S, Malipatil S, Foo MS, Burrow MF, Thomas D (2007) Resin bonding using an all-etch or self-etch adhesive to enamel after carbamide peroxide and/or CPP-ACP treatment. Aust Dent J 52:133–137. https://doi.org/10.1111/j.1834-7819,2007.tb00478.x
El-din AN, Miller B, Griggs J, Wakefeld C (2006) Immediate bonding to bleached enamel. Oper Dent 31:106–114. https://doi.org/10.2341/04-201
Gurgan S, Alpaslan T, Kiremitci A, Cakir FY, Yazıcı E, Gorucu J (2009) Efect of diferent adhesive systems and laser treatment on the shear bond strength of bleached enamel. J Dent 37:527–534. https://doi.org/10.1016/j.jdent.2009.03.012
Funding
This study was funded by the Technological and Scientific Council of Turkey (TUBITAK) (project number:1139B411).
Author information
Authors and Affiliations
Contributions
Ekrikaya Semiha: data curation, formal analysis, investigation, methodology, resources, software, supervision, validation, visualization, writing – original draft, writing – review & editing. Yilmaz Ebubekir: methodology, writing – original draft, writing – review & editing. Arslan Soley: methodology, writing – original draft, writing – review & editing. Karaaslan Rabia: investigation, methodology, writing – original draft, writing – review & editing. Ildiz Nilay: methodology, writing – original draft, writing – review & editing. Celik Cagla: conceptualization, formal analysis, investigation. Ocsoy Ismail: conceptualization, formal analysis, the investigation, methodology, resources, software, supervision, validation, visualization, writing – original draft, writing – review & editing. all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Ethics approval
This study protocol was approved by the Nuh Naci Yazgan University Ethics Committee (2022/003–001).
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ekrikaya, S., Yilmaz, E., Arslan, S. et al. Dentin bond strength and antimicrobial activities of universal adhesives containing silver nanoparticles synthesized with Rosa canina extract. Clin Oral Invest 27, 6891–6902 (2023). https://doi.org/10.1007/s00784-023-05306-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-023-05306-6