Abstract
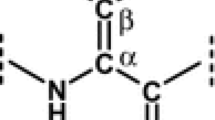
Naturally occurring secondary amino acids, with proline as the main representative, contain an alpha-imino group in a cycle that is typically four-, five-, and six-membered. The unique ring structure exhibits exceptional properties—conformational rigidity, chemical stability, and specific roles in protein structure and folding. Many proline analogues have been used as valuable compounds for the study of metabolism of both prokaryotic and eukaryotic cells and for the synthesis of compounds with desired biological, pharmaceutical, or industrial properties. The d-forms of secondary amino acids play different roles in living organisms than the l-forms. They have different metabolic pathways, biological, physiological, and pharmacological effects, they can be indicators of changes and also serve as biomarkers of diseases. In the scientific literature, the number of articles examining d-amino acids in biological samples is increasing. The review summarises information on the occurrence and importance of d- and l-secondary amino acids—azetidic acid, proline, hydroxyprolines, pipecolic, nipecotic, hydroxypipecolic acids and related peptides containing these d-AAs, as well as the main analytical methods (mostly chromatographic) used for their enantiomeric determination in different matrices (biological samples, plants, food, water, and soil).




Similar content being viewed by others
Abbreviations
- 2D-PC:
-
Two-dimensional paper chromatography
- 2D-HPLC:
-
Two-dimensional high-performance liquid chromatography
- 3c-L-Hyp:
-
cis-3-Hydroxy-l-proline, ((2S,3R)-3-Hydroxypyrrolidine-2-carboxylic acid)
- 3t-L-Hyp:
-
trans-3-Hydroxy-l-proline, ((2S,3S)-3-Hydroxypyrrolidine-2-carboxylic acid)
- 3c-D-Hyp:
-
cis-3-Hydroxy-d- proline ((2R,3S)-3-hydroxypyrrolidine-2-carboxylic acid)
- 3t-D-Hyp:
-
trans-3-Hydroxy-d- proline ((2R,3R)-3-hydroxypyrrolidine-2-carboxylic acid)
- 4c-L-Hyp:
-
cis-4-Hydroxy-l- proline ((2S,4S)-4-Hydroxypyrrolidine-2-carboxylic acid)
- 4t-L-Hyp:
-
trans-4-Hydroxy-l- proline ((2S,4R)-4-hydroxypyrrolidine-2-carboxylic acid)
- 4c-D-Hyp:
-
cis-4-Hydroxy-d- proline ((2R,4R)-4-Hydroxypyrrolidine-2-carboxylic acid)
- 4t-D-Hyp:
-
trans-4-Hydroxy-d- proline ((2R,4S)-4-Hydroxypyrrolidine-2-carboxylic acid)
- 3c-L-HyPip:
-
cis-3-Hydroxy-l-pipecolic acid ((2S, 3R)-3-Hydroxypiperidine-2-carboxylic acid)
- 3t-L-HyPip:
-
trans-3-Hydroxy-l- pipecolic acid ((2S,3S)-3-Hydroxypiperidine-2-carboxylic acid)
- 3c-D-HyPip:
-
cis-3-Hydroxy-d- pipecolic acid ((2R, 3S)-3-Hydroxypiperidine-2-carboxylic acid)
- 3t-D-HyPip:
-
trans-3-Hydroxy-d- pipecolic acid ((2R, 3R)-3-Hydroxypiperidine-2-carboxylic
- 4c-L-HyPip:
-
cis-4-Hydroxy-l- pipecolic acid ((2S,4R)-4-Hydroxypiperidine-2-carboxylic acid)
- 4t-L-HyPip:
-
trans-4-Hydroxy-l- pipecolic acid ((2S,4S)-4-Hydroxypiperidine-2-carboxylic acid)
- 4c-D-HyPip:
-
cis-4-Hydroxy-d- pipecolic acid ((2R,4S)-4-Hydroxypiperidine-2-carboxylic acid)
- 4t-D-HyPip:
-
trans-4-Hydroxy-d- pipecolic acid ((2R,4R)-4-Hydroxypiperidine-2-carboxylic acid)
- 5c-L-HyPip:
-
cis-5-Hydroxy-l- pipecolic acid ((2S,5S)-5-Hydroxypiperidine-2-carboxylic acid)
- 5t-L-HyPip:
-
trans-5-Hydroxy-l- pipecolic acid ((2S,5R)-5-Hydroxypiperidine-2-carboxylic acid)
- AA(s):
-
Amino acid(s)
- AcCl:
-
Acetyl chloride
- AQC:
-
6-Aminoquinolyl-N-hydroxysuccinimidyl carbamate
- AMBI:
-
1-Phenylethylisothiocyanate, alpha-Methylbenzyl isothiocyanate
- BSTFA:
-
N,O-Bis(trimethylsilyl)trifluoroacetamide
- CLEC:
-
Chiral ligand-exchange chromatography
- CSP:
-
Chiral stationary phase
- DAD:
-
Diode array detector
- DCl:
-
: Deuterium chloride
- ddY/DAO:
-
Mutant strain lacking d-Amino acid oxidase activity
- diHyPip:
-
Dihydroxy pipecolic acid
- DMT-(S)-Pro-OSu:
-
(S)-2,5-Dioxopyrrolidin-1-yl-1-(4,6-dimethoxy-1,3,5-triazin-2-yl) pyrrolidine-2-carboxylate
- D-Aze:
-
d-Azetidic acid, d-Azetidine-2-carboxylic acid, ((2R)-Azetidine-2-carboxylic acid)
- L-Aze:
-
l- Azetidic acid, l-Azetidine-2-carboxylic acid, ((2S)-Azetidine-2-carboxylic acid)
- D-Nip:
-
d-Nipecotic acid, ((3R)-Piperidine-3-carboxylic acid)
- L-Nip:
-
l- Nipecotic acid, ((3S)-Piperidine-3-carboxylic acid)
- D-Pip:
-
d-Pipecolic acid, ((2R)-Piperidine-2-carboxylic acid, d-Homoproline)
- L-Pip:
-
l- Pipecolic acid, ((2S)-Piperidine-2-carboxylic acid, l-Homoproline)
- D-Pro:
-
d- Proline, ((2R)-Pyrrolidine-2-carboxylic acid)
- L-Pro:
-
l- Proline, ((2S)-Pyrrolidine-2-carboxylic acid)
- EC:
-
Enzyme Commission number
- EtOD:
-
Ethanol-OD
- FDAA:
-
Marfey’s reagent, 1-Fluoro-2,4-dinitrophenyl-5-l-alanine amide
- FDVA:
-
Nα -(2,4-dinitro-5-fluorophenyl)-d or l-valine amide
- FDLA:
-
Nα -(2,4-dinitro-5-fluorophenyl)-d or l-leucine amide
- FITC:
-
Fluorescein isothiocyanate
- FoPip4H:
-
l-Pip trans-4-hydroxylase of Fusarium oxysporum
- FLEC:
-
1-(9-Fluorenyl)ethyl chloroformate
- FMOC-Cl:
-
9-Fluorenylmethoxycarbonyl chloride, 9-Fluorenylmethyl chloroformate
- GABA:
-
γ-Amino butyric acid
- GC/FID:
-
Gas chromatography with flame ionization detector
- GC/MS:
-
Gas chromatography with mass spectrometric detector
- GITC:
-
2,3,4,6-Tetra-O-acetyl-β -d-glucopyranosyl isothiocyanate
- HFBCF:
-
2,2,3,3,4,4,4-Heptafluorobutyl chloroformate
- Hyp(s):
-
Hydroxyproline (s)
- HyPip(s):
-
Hydroxypipecolic acid (s)
- HPLC:
-
High-performance liquid chromatography
- HPLC/DAD:
-
HPLC with diode array detector
- HPLC/Fl:
-
HPLC with fluorescence detector
- HPLC/UV:
-
HPLC with UV detector
- IBDC:
-
N-Isobutyryl-d-cysteine
- IBLC:
-
N-Isobutyryl-l-cysteine
- IRMPD:
-
Infrared multiple photon dissociation spectroscopy
- HPLC/MS:
-
Liquid chromatography–mass spectrometry
- HPLC/MSMS:
-
Liquid chromatography–tandem mass spectrometry
- MSTFA:
-
N-Methyl-N-(trimethylsilyl)trifluoroacetamide
- NBD-F:
-
4-Fluoro-7-nitro-2,1,3-benzoxadiazole
- NBD-PyNCS:
-
4-(3-Isothiocyanatopyrrolidin-1-yl)-7-nitrobenzofurazan
- N-HyPip:
-
N-Hydroxypipecolic acid, 1-Hydroxypiperidine-2-carboxylic acid
- NIFE:
-
N-(4-Nitrophenoxycarbonyl)-l-phenylalanine-2-methoxyethyl ester
- NMR:
-
Nuclear magnetic resonance
- OPA:
-
o-Phthaldialdehyde
- PFPA:
-
Pentafluoropropionic anhydride
- P5C:
-
Pyrroline-5-carboxylate
- PCA:
-
Pyroglutamic acid
- R(-)-DBD-PyNCS:
-
R(-)-4-(3-Isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-bezoxadiazole
- S( +)-DBD-PyNCS:
-
S( +)-4-(3-Isothiocyanatopyrrolidin-1-yl)-7-(N,N-dimethylaminosulfonyl)-2,1,3-bezoxadiazole
- SFC/MS:
-
Supercritical fluid chromatography–mass spectrometry
- SPE:
-
Solid phase extraction
- TFA:
-
Trifluoroacetic anhydride
- TLC:
-
Thin-layer chromatography
- UPLC:
-
Ultra-performance liquid chromatography
References
Abe I, Nakahara T (1996) Enantiomer separation of amino acids as their N-alkyloxycarbonyl alkylamide derivatives by chiral phase capillary GC. HRCJ High Resol Chromatogr 19(9):511–514
Abe I, Kawazuma M, Fujimoto N, Nakahara T (1995) N-alkyloxycarbonyl isobutylamides as readily prepared diamide derivatives of amino-acids for separation of enantiomeric isomers by chiral phase capillary gas-chromatography. Chem Lett 4:329–330. https://doi.org/10.1246/cl.1995.329
Abe I, Minami H, Nakao Y, Nakahara T (2002) N-pivaloyl methyl ester derivatives of amino acids for separation of enantiomers by chiral-phase capillary gas chromatography. J Sep Sci 25(10–11):661–664. https://doi.org/10.1002/1615-9314(20020701)25:10/11%3c661::aid-jssc661%3e3.0.co;2-x
Aher RD, Sudalai A (2016) Proline catalyzed enantioselective synthesis of (2S,3S)-3-hydroxypipecolic acid and formal synthesis of (+)-swainsonine. Tetrahedron Lett 57(19):2021–2024. https://doi.org/10.1016/j.tetlet.2016.03.042
Aiello A, Fattorusso E, Giordano A, Menna M, Muller WEG, Perovic-Ottstadt S, Schroder HC (2007) Damipipecolin and damituricin, novel bioactive bromopyrrole alkaloids from the Mediterranean sponge Axinella damicornis. Bioorg Med Chem 15(17):5877–5887. https://doi.org/10.1016/j.bmc.2007.05.074
Ali HSM, Patzold R, Brückner H (2010) Gas chromatographic determination of amino acid enantiomers in bottled and aged wines. Amino Acids 38(3):951–958. https://doi.org/10.1007/s00726-009-0304-1
Ali HSM, Alhaj OA, Al-Khalifa AS, Brückner H (2014) Determination and stereochemistry of proteinogenic and non-proteinogenic amino acids in Saudi Arabian date fruits. Amino Acids 46(9):2241–2257. https://doi.org/10.1007/s00726-014-1770-7
Arbor S, Kao J, Wu Y, Marshall GR (2008) c[D-pro-Pro-D-pro-N-Methyl-Ala] adopts a rigid conformation that serves as a scaffold to mimic reverse-turns. Biopolymers 90(3):384–393. https://doi.org/10.1002/bip.20869
Armstrong DW, Gasper M, Lee SH, Zukowski J, Ercal N (1993a) D-amino-acid levels in human physiological fluids. Chirality 5(5):375–378. https://doi.org/10.1002/chir.530050519
Armstrong DW, Gasper MP, Lee SH, Ercal N, Zukowski J (1993b) Factors controlling the level and determination of D-amino acids in the urine and plasma of laboratory rodents. Amino Acids 5(2):299–315. https://doi.org/10.1007/bf00805992
Armstrong DW, Zukowski J, Ercal N, Gasper M (1993c) Stereochemistry of pipecolic acid found in the urine and plasma of subjects with peroxisomal deficiencies. J Pharm Biomed Anal 11(10):881–886. https://doi.org/10.1016/0731-7085(93)80044-2
Bach TMH, Takagi H (2013) Properties, metabolisms, and applications of L-proline analogues. Appl Microbiol Biot 97(15):6623–6634. https://doi.org/10.1007/s00253-013-5022-7
Baek SH, Lee JG, Park SY, Piao XL, Kim HY, Bae ON, Park JH (2012) Gas chromatographic determination of azetidine-2-carboxylic acid in rhizomes of Polygonatum sibiricum and Polygonatum odoratum. J Food Compos Anal 25(2):137–141. https://doi.org/10.1016/j.jfca.2011.09.005
Barbaro E, Zangrando R, Vecchiato M, Turetta C, Barbante C, Gambaro A (2014) D- and L-amino acids in Antarctic lakes: assessment of a very sensitive HPLC-MS method. Anal Bioanal Chem 406(22):5259–5270. https://doi.org/10.1007/s00216-014-7961-y
Berthod A, Liu YB, Bagwill C, Armstrong DW (1996) Facile liquid chromatographic enantioresolution of native amino acids and peptides using a teicoplanin chiral stationary phase. J Chromatogr A 731(1–2):123–137
Bertrand M, Chabin A, Brack A, Westall F (2008) Separation of amino acid enantiomers via chiral derivatization and non-chiral gas chromatography. J Chromatogr A 1180(1–2):131–137. https://doi.org/10.1016/j.chroma.2007.12.004
Bhushan R, Kumar R (2009) Analysis of multicomponent mixture and simultaneous enantioresolution of proteinogenic and non-proteinogenic amino acids by reversed-phase high-performance liquid chromatography using chiral variants of Sanger’s reagent. Anal Bioanal Chem 394(6):1697–1705. https://doi.org/10.1007/s00216-009-2854-1
Bleecker AB, Romeo JT (1981) 2,4-trans-4,5-trans-4,5-Dihydroxypipecolic acid and cis-5-hydroxypipecolicacid from leaves of Calliandra-angustifolia and sap of Calliandra-confusa. Phytochemistry 20(8):1845–1846. https://doi.org/10.1016/0031-9422(81)84017-4
Botman PNM, Dommerholt FJ, de Gelder R, Broxterman QB, Schoemaker HE, Rutjes F, Blaauw RH (2004) Diastereoselective synthesis of (2S,5R)-5-hydroxypipecolicacid and 6-substituted derivatives. Org Lett 6(26):4941–4944. https://doi.org/10.1021/ol047774v
Brandi L, Lazzarini A, Cavaletti L, Abbondi M, Corti E, Ciciliato I, Gastaldo L, Marazzi A, Feroggio M, Fabbretti A, Maio A, Colombo L, Donadio S, Marinelli F, Losi D, Gualerzi CO, Selva E (2006) Novel tetrapeptide inhibitors of bacterial protein synthesis produced by a Streptomyces sp. Biochemistry 45(11):3692–3702. https://doi.org/10.1021/Bi052540K
Brenner SA, Romeo JT (1986) Fungitoxic effects of nonprotein imino acids on growth of saprophytic fungi isolated from the leaf surface of Calliandra-Haematocephala. Appl Environ Microbiol 51(4):690–693. https://doi.org/10.1128/aem.51.4.690-693.1986
Brückner H, Hausch M (1989) Detection of free D-amino acids in food by chiral phase capillary gas-chromatography. HRC J High Resol Chromatogr 12(10):680–684
Brückner H, Hausch M (1993) Gas-chromatographic characterization of free D-amino acids in the blood-serum of patients with renal disorders and of healthy-volunteers. J Chromatogr Biomed Appl 614(1):7–17. https://doi.org/10.1016/0378-4347(93)80218-s
Brückner H, Lüpke M (1991) Determination of amino-acid enantiomers in orange juices by chiral phase capillary gas-chromatography. Chromatographia 31(3–4):123–128. https://doi.org/10.1007/bf02274558
Brückner H, Schieber A (2001) Determination of amino acid enantiomers in human urine and blood serum by gas chromatography-mass spectrometry. Biomed Chromatogr 15(3):166–172. https://doi.org/10.1002/bmc.57
Brückner H, Westhauser T (2003) Chromatographic determination of L- and D-amino acids in plants. Amino Acids 24(1–2):43–55. https://doi.org/10.1007/s00726-002-0322-8
Brückner H, Justus J, Kirschbaum J (2001) Saccharide induced racemization of amino acids in the course of the Maillard reaction. Amino Acids 21(4):429–433. https://doi.org/10.1007/s007260170007
Bungo T, Izumi T, Kawamura K, Takagi T, Ueda H, Furuse M (2003) Intracerebroventricular injection of muscimol, baclofen or nipecotic acid stimulates food intake in layer-type, but not meat-type, chicks. Brain Res 993(1–2):235–238. https://doi.org/10.1016/j.brainres.2003.09.017
Byun S, Park HJ, Joo JC, Kim YH (2019) Enzymatic Synthesis of D-pipecolic acid by engineering the substrate specificity of trypanosoma cruzi proline racemase and its molecular docking study. Biotechnol Bioprocess Eng 24(1):215–222. https://doi.org/10.1007/s12257-018-0367-5
Calabrese M, Stancher B (1999) A study of the proline isomerisation in typical Italian wines. J Sci Food Agric 79(11):1357–1360. https://doi.org/10.1002/(sici)1097-0010(199908)79:11%3c1357::aid-jsfa371%3e3.0.co;2-3
Carotti A, Ianni F, Camaioni E, Pucciarini L, Marinozzi M, Sardella R, Natalini B (2017) N-Decyl-S-trityl-(R)-cysteine, a new chiral selector for “green” ligand-exchange chromatography applications. J Pharm Biomed Anal 144:31–40. https://doi.org/10.1016/j.jpba.2017.02.009
Chabrun F, Dieu X, Rousseau G, Chupin S, Letournel F, Procaccio V, Bonneau D, Lenaers G, Simard G, Mirebeau-Prunier D, de la Barca JMC, Reynier P (2020) Metabolomics reveals highly regional specificity of cerebral sexual dimorphism in mice. Prog Neurobiol. https://doi.org/10.1016/j.pneurobio.2019.101698
Chalmers DK, Marshall GR (1995) Pro-D-NMe-amino acid and D-Pro-NMe-amino acid - simple, efficient reverse-turn constraints. J Am Chem Soc 117(22):5927–5937. https://doi.org/10.1021/ja00127a004
Chang YF (1978) Lysine metabolism in rat brain: pipecolic acid forming pathway. J Neurochem 30(2):347–354. https://doi.org/10.1111/j.1471-4159.1978.tb06536.x
Chavan SP, Khairnar LB, Pawar KP, Chavan PN, Kawale SA (2015) Enantioselective syntheses of (R)-pipecolicacid, (2R,3R)-3-hydroxypipecolic acid, beta-(+)-conhydrine and (-)-swainsonine using an aziridine derived common chiral synthon. RSC Adv 5(62):50580–50590. https://doi.org/10.1039/c5ra06429e
Chavan SP, Kadam AL, Shinde SS, Gonnade RG (2020) Furan-derived chiral bicycloaziridino lactone synthon: collective syntheses of oseltamivir phosphate (Tamiflu), (S)-pipecolic acid and its 3-hydroxy derivatives. Chem Asian J 15(3):415–424. https://doi.org/10.1002/asia.201901523
Chen YC, Holmes EC, Rajniak J, Kim JG, Tang S, Fischer CR, Mudgett MB, Sattely ES (2018) N-hydroxy-pipecolic acid is a mobile metabolite that induces systemic disease resistance in Arabidopsis. Proc Natl Acad Sci USA 115(21):E4920–E4929. https://doi.org/10.1073/pnas.1805291115
Cherkin A, Vanharreveld A (1978) L-proline and related compounds - correlation of structure, amnesic potency and anti-spreading depression potency. Brain Res 156(2):265–273. https://doi.org/10.1016/0006-8993(78)90508-5
Cherkin A, Davis JL, Garman MW (1978) D-proline - stereospecificity and sodium-chloride dependence of lethal convulsant activity in chick. Pharmacol Biochem Behav 8(5):623–625. https://doi.org/10.1016/0091-3057(78)90399-4
Chiavaro E, Caligiani A, Palla G (1998) Chiral indicators of ageing in balsamic vinegars of modena. Ital J Food Sci 10(4):329–337
Cho ES, Sung JY, Jin JS, Hyun MH (2018) Liquid chromatographic resolution of proline and pipecolic acid derivatives on chiral stationary phases based on (+)-(18-crown-6)-2,3,11,12-tetracarboxylic acid. J Sep Sci 41(6):1192–1198. https://doi.org/10.1002/jssc.201700996
Cimlová J, Kružberská P, Švagera Z, Hušek P, Šimek P (2012) In situ derivatization-liquid liquid extraction as a sample preparation strategy for the determination of urinary biomarker prolyl-4-hydroxyproline by liquid chromatography-tandem mass spectrometry. J Mass Spectrom 47(3):294–302. https://doi.org/10.1002/jms.2952
Clarklewis JW, Mortimer PI (1959) Occurrence of 4-hydroxypipecolic acid in Acacia species. Nature 184(4694):1234–1235. https://doi.org/10.1038/1841234b0
Clarklewis J, Mortimer PI (1961) 4-Hydroxypipecolic acid from Acacia species, and its stereoisomers. J Chem Soc (JAN). https://doi.org/10.1039/jr9610000189
Coatnoan N, Berneman A, Chamond N, Minoprio P (2009) Proline racemases: insights into Trypanosoma cruzi peptides containing D-proline. Mem Inst Oswaldo Cruz 104:295–300. https://doi.org/10.1590/s0074-02762009000900039
Cochi A, Pardo DG, Cossy J (2013) Synthesis of 3-hydropipecolic acids. Eur J Org Chem 5:809–829. https://doi.org/10.1002/ejoc.201201415
Codini M, Palmerini CA, Fini C, Lucarelli C, Floridi A (1991) High-performance liquid-chromatographic method for the determination of prolyl peptides in urine. J Chromatogr 536(1–2):337–341. https://doi.org/10.1016/s0021-9673(01)89267-0
Couty F (1999) Asymmetric syntheses of piecolic acid and derivatives. Amino Acids 16(3–4):297–320. https://doi.org/10.1007/bf01388174p
Datta D, Harikrishna A, Nagaraj R, Chaudhary N (2019) Self-assembly of beta-turn motif-connected tandem repeats of A beta(16–22) and its aromatic analogs. Peptide Sci. https://doi.org/10.1002/pep2.24099
Davankov VA, Rogozhin SV (1971) Ligand chromatography as a novel method for investigation of mixed complexes - stereoselective effects in alpha-amino acid copper(ii) complexes. J Chromatogr 60(2):280–290
Despontin J, Marlier M, Dardenne G (1977) L-cis-5-hydroxy pipecolicacid from seeds of gymnocladus-dioicus. Phytochemistry 16(3):387–388. https://doi.org/10.1016/0031-9422(77)80070-8
Dhar TGM, Borden LA, Tyagarajan S, Smith KE, Branchek TA, Weinshank RL, Gluchowski C (1994) Design, synthesis and evaluation of substituted triarylnipecotic acid-derivatives as GABA uptake inhibitors - identification of a ligand with moderate affinity and selectivity for the cloned human GABA transporter GAT-3. J Med Chem 37(15):2334–2342. https://doi.org/10.1021/jm00041a012
Di Natale C, La Manna S, Avitabile C, Florio D, Morelli G, Netti PA, Marasco D (2020) Engineered beta-hairpin scaffolds from human prion protein regions: Structural and functional investigations of aggregates (vol 96, 103594, 2020). Bioorg Chem. https://doi.org/10.1016/j.bioorg.2020.103853
Du S, Wang Y, Weatherly CA, Holden K, Armstrong DW (2018) Variations of L- and D-amino acid levels in the brain of wild-type and mutant mice lacking D-amino acid oxidase activity. Anal Bioanal Chem 410(12):2971–2979. https://doi.org/10.1007/s00216-018-0979-9
Dziewiatkowski DD, Hascall VC, Riolo RL (1972) Epimerization of trans-4-hydroxy-L- prolin to cis-4-hydroxy-D- prolin during acid-hydrolysis of collagen. Analyt Biochem 49(2):550. https://doi.org/10.1016/0003-2697(72)90461-7
Egli J, Schnitzer T, Dietschreit JCB, Ochsenfeld C, Wennemers H (2020) Why proline? Influence of ring-size on the collagen triple helix. Org Lett 22(2):348–351. https://doi.org/10.1021/acs.orglett.9b03528
Einarsson S, Josefsson B, Moller P, Sanchez D (1987) Separation of amino-acid enantiomers and chiral amines using precolumn derivatization with (+)-1-(9-fluorenyl)ethyl chloroformate and reversed-phase liquid-chromatography. Anal Chem 59(8):1191–1195. https://doi.org/10.1021/ac00135a025
Ekborgott KH, Armstrong DW (1996) Evaluation of the concentration and enantiomeric purity of selected free amino acids in fermented malt beverages (beers). Chirality 8(1):49–57. https://doi.org/10.1002/(sici)1520-636x(1996)8:1%3c49::aid-chir10%3e3.0.co;2-s
Erbe T, Brückner H (1998) Chiral amino acid analysis of vinegars using gas chromatography - selected ion monitoring mass spectrometry. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung Food Res Technol 207(5):400–409. https://doi.org/10.1007/s002170050352
Erbe T, Brückner H (2000) Studies on the optical isomerization of dietary amino acids in vinegar and aqueous acetic acid. Eur Food Res Technol 211(1):6–12. https://doi.org/10.1007/s002170050581
Fichman Y, Gerdes SY, Kovacs H, Szabados L, Zilberstein A, Csonka LN (2015) Evolution of proline biosynthesis: enzymology, bioinformatics, genetics, and transcriptional regulation. Biol Rev 90(4):1065–1099. https://doi.org/10.1111/brv.12146
Fleischer H, Thurow K (2011) Rapid enantiomeric excess determination of D- and L- Proline using electrospray ionization-mass spectrometry. Am Labor 43(9):32
Fleischer H, Thurow K (2013) Fast mass spectrometry-based enantiomeric excess determination of proteinogenic amino acids. Amino Acids 44(3):1039–1051. https://doi.org/10.1007/s00726-012-1439-z
Fouche M, Schafer M, Berghausen J, Desrayaud S, Blatter M, Piechon P, Dix I, Garcia AM, Roth HJ (2016) Design and development of a cyclic decapeptide scaffold with suitable properties for bioavailability and oral exposure. ChemMedChem 11(10):1048–1059. https://doi.org/10.1002/cmdc.201600082
Fowden L (1955) Azetidine-2-carboxylic acid -new constituent of plants. Nature 176(4477):347–348. https://doi.org/10.1038/176347a0
Fowden L (1956) Azetidine-2-carboxylic acid - new cyclic imino acid occurring in plants. Biochem J 64(2):323–332. https://doi.org/10.1042/bj0640323
Fowden L (1960) The metabolism of labelled lysine and pipecolic acid by Acacia Phyllodes. J Exp Bot 11(33):302–315. https://doi.org/10.1093/jxb/11.3.302
Fowden L (1963) Amino-acid analogues and growth of seedlings. J Experim Botany 14(42):387. https://doi.org/10.1093/jxb/14.3.387
Fowden L, Richmond MH (1963) Replacement of proline by azetidine-2-carboxylic acid during biosynthesis of protein. Biochem Biophys Acta 71(2):459. https://doi.org/10.1016/0006-3002(63)91104-1
Frenkel J, Wess C, Vyverman W, Pohnert G (2014) Chiral separation of a diketopiperazine pheromone from marine diatoms using supercritical fluid chromatography. J Chromatogr B Anal Technol Biomed Life Sci 951:58–61. https://doi.org/10.1016/j.jchromb.2013.12.040
Friedman M (1999) Chemistry, nutrition, and microbiology of D-amine acids. J Agric Food Chem 47(9):3457–3479. https://doi.org/10.1021/jf990080u
Fujii T, Mukaihara M, Agematu H, Tsunekawa H (2002) Biotransformation of L-lysine to L-pipecolic acid catalyzed by L-lysine 6-aminotransferase and pyrroline-5-carboxylate reductase. Biosci Biotechnol Biochem 66(3):622–627. https://doi.org/10.1271/bbb.66.622
Fujita T, Amuro Y, Hada T, Higashino K (1999a) Plasma levels of pipecolic acid, both L- and D-enantiomers, in patients with chronic liver diseases, especially hepatic encephalopathy. Clin Chim Acta 287(1–2):99–109. https://doi.org/10.1016/s0009-8981(99)00123-0
Fujita T, Hada T, Higashino K (1999b) Origin of D- and L-pipecolic acid in human physiological fluids: a study of the catabolic mechanism to pipecolic acid using the lysine loading test. Clin Chim Acta 287(1–2):145–156. https://doi.org/10.1016/s0009-8981(99)00129-1
Fujita T, Fujita M, Kodama T, Hada T, Higashino K (2003) Determination of D- and L-pipecolic acid in food samples including processed foods. Ann Nutr Metab 47(3–4):165–169. https://doi.org/10.1159/000070040
Futamura Y, Kurokawa M, Obata R, Nishiyama S, Sugai T (2005) Efficient route to (S)-azetidine-2-carboxylic acid. Biosci Biotechnol Biochem 69(10):1892–1897. https://doi.org/10.1271/bbb.69.1892
Gadais C, Van holsbeeck K, Moors SLC, Buyst D, Feher K, Van Hecke K, Tourwe D, Brigaud T, Martin C, De Proft F, Pytkowicz J, Martins JC, Chaume G, Ballet S (2019) Trifluoromethylated proline surrogates as part of “Pro-Pro” turn-inducing templates. ChemBioChem 20(19):2513–2518. https://doi.org/10.1002/cbic.201900294
Galaverna G, Corradini R, Demunari E, Dossena A, Marchelli R (1993) Chiral separation of unmodified amino-acids by ligand-exchange high-performance liquid-chromatography using copper(Ii) complexes of L-amino-acid amides as additives to the eluent. J Chromatogr A 657(1):43–54
Galaverna G, Corradini R, Dallavalle F, Folesani G, Dossena A, Marchelli R (2001) Chiral separation of amino acids by copper(II) complexes of tetradentate diaminodiamido-type ligands added to the eluent in reversed-phase high-performance liquid chromatography: a ligand exchange mechanism. J Chromatogr A 922(1–2):151–163
Ganesan MS, Raja KK, Narasimhan K, Murugesan S, Kumar BK (2020) Design, synthesis, alpha-amylase inhibition and in silico docking study of novel quinoline bearing proline derivatives. J Mol Struct. https://doi.org/10.1016/j.molstruc.2020.127873
Gao XZ, Ma QY, Zhu HL (2015) Distribution, industrial applications, and enzymatic synthesis of D-amino acids. Appl Microbiol Biotechnol 99(8):3341–3349. https://doi.org/10.1007/s00253-015-6507-3
Gauza-Mlodarczyk M, Kubisz L, Wlodarczyk D (2017) Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int J Biol Macromol 104:987–991. https://doi.org/10.1016/j.ijbiomac.2017.07.013
Gogami Y, Okada K, Oikawa T (2011) High-performance liquid chromatography analysis of naturally occurring D-amino acids in sake. J Chromatogr B-Analyt Technol Biomed Life Sci 879(29):3259–3267. https://doi.org/10.1016/j.jchromb.2011.04.006
Gordes D, Kolukisaoglu U, Thurow K (2011) Uptake and conversion of d-amino acids in Arabidopsis thaliana. Amino Acids 40(2):553–563. https://doi.org/10.1007/s00726-010-0674-4
Greck C, Ferreira F, Genet JP (1996) Synthesis of both enantiomers of trans 3-hydroxypipecolic acid. Tetrahedron Lett 37(12):2031–2034. https://doi.org/10.1016/0040-4039(96)00191-8
Grobbelaar N, Pollard JK, Steward FC (1955) New soluble nitrogen compounds (amino-acids and imino-acids and amides) in plants. Nature 175(4460):703–708. https://doi.org/10.1038/175703a0
Haddad JJE, Lauterbach R, Saade NE, Safieh-Garabedian B, Land SC (2001) alpha-Melanocyte-related tripeptide, Lys-D-Pro-Val, ameliorates endotoxin-induced nuclear factor kappa B translocation and activation: evidence for involvement of an interleukin-1 beta(193–195) receptor antagonism in the alveolar epithelium. Biochem J 355:29–38. https://doi.org/10.1042/bj3550029
Hadjistasi CA, Stavrou IJ, Stefan-Van Staden RI, Aboul-Enein HY, Kapnissi-Christodoulou CP (2013) Chiral separation of the clinically important compounds fucose and pipecolic acid using CE: determination of the most effective chiral selector. Chirality 25(9):556–560. https://doi.org/10.1002/chir.22170
Hamase K (2007) Sensitive two-dimensional determination of small amounts of D-amino acids in mammals and the study on their functions. Chem Pharm Bull 55(4):503–510. https://doi.org/10.1248/cpb.55.503
Hamase K, Morikawa A, Etoh S, Tojo Y, Miyoshi Y, Zaitsu K (2009) Analysis of small amounts of D-amino acids and the study of their physiological functions in mammals. Anal Sci 25(8):961–968
Hamasu K, Shigemi K, Tsuneyoshi Y, Yamane H, Sato H, Denbow DM, Furuse M (2010) Intracerebroventricular injection of L-proline and D-proline induces sedative and hypnotic effects by different mechanisms under an acute stressful condition in chicks. Amino Acids 38(1):57–64. https://doi.org/10.1007/s00726-008-0204-9
Hara R, Kino K (2020) Enzymatic reactions and microorganisms producing the various isomers of hydroxyproline. Appl Microbiol Biotechnol 104(11):4771–4779. https://doi.org/10.1007/s00253-020-10603-1
Hartmann M, Zeier J (2018) L-lysine metabolism to N-hydroxypipecolic acid: an integral immune-activating pathway in plants. Plant J 96(1):5–21. https://doi.org/10.1111/tpj.14037
Hartmann M, Zeier J (2019) N-hydroxypipecolic acid and salicylic acid: a metabolic duo for systemic acquired resistance. Curr Opin Plant Biol 50:44–57. https://doi.org/10.1016/j.pbi.2019.02.006
Hartmann M, Zeier T, Bernsdorff F, Reichel-Deland V, Kim D, Hohmann M, Scholten N, Schuck S, Brautigam A, Holzel T, Ganter C, Zeier J (2018) Flavin monooxygenase-generated N-hroxypipecolic acid is a critical element of plant systemic immunity. Cell 173(2):456. https://doi.org/10.1016/j.cell.2018.02.049
Hassid E, Applebaum SW, Birk Y (1976) Azetidine-2-carboxylic acid - naturally occurring inhibitor of Spodoptera -Littoralis -(Boisd) (Lepidoptera-Noctuidae). Phytoparasitica 4(3):173–183. https://doi.org/10.1007/bf02981084
Hatanaka SI, Kaneko S (1977) Cis-5-hydroxy-L-pipecolic acid from Morus-Alba and Lathyrus-Japonicus. Phytochemistry 16(7):1041–1042. https://doi.org/10.1016/s0031-9422(00)86718-7
Hedges JB, Ryan KS (2020) Biosynthetic pathways to nonproteinogenic alpha-amino acids. Chem Rev 120(6):3161–3209. https://doi.org/10.1021/acs.chemrev.9b00408
Hegarty MP (1957) The isolation and identification of 5-hydroxypiperidine-2-carboxylic acid from Leucaena -Glauca Benth. Australian J Chemy 10(4):484–488. https://doi.org/10.1071/ch9570484
Hibi M, Mori R, Miyake R, Kawabata H, Kozono S, Takahashi S, Ogawa J (2016) Novel enzyme family found in filamentous fungi catalyzing trans-4-hydroxylation of L-pipicolic acid. Appl Environ Microbiol 82(7):2070–2077. https://doi.org/10.1128/aem.03764-15
Himaja M, Ranjitha A, Mali SV (2012) Synthesis, docking and anticancer activity studies of D-proline-incorporated wainunuamide. J Chem Sci 124(5):1049–1055. https://doi.org/10.1007/s12039-012-0301-x
Hinko CN, Crider AM, Wood JD (1988) A comparison of prodrug esters of nipecotic acid. Neuropharmacology 27(5):475–483. https://doi.org/10.1016/0028-3908(88)90129-3
Hoarau S, Fauchere JL, Pappalardo L, Roumestant ML, Viallefont P (1996) Synthesis of enantiomerically pure (2R, 5S)- and (2R, 5R)-5-hydroxypipecolic acid from glycinate Schiff bases. Tetrahedron-Asymmetry 7(9):2585–2593. https://doi.org/10.1016/0957-4166(96)00332-1
Hoffmann CV, Pell R, Lammerhofer M, Lindner W (2008) Synergistic effects on enantioselectivity of zwitterionic chiral stationary phases for separations of chiral acids, bases, and amino acids by HPLC. Anal Chem 80(22):8780–8789
Holmes EC, Chen YC, Sattely ES, Mudgett MB (2019) An engineered pathway for N-hydroxy-pipecolic acid synthesis enhances systemic acquired resistance in tomato. Sci Signal. https://doi.org/10.1126/scisignal.aay3066
Hook DJ, Vining LC (1973) Biosynthetic precursors of etamycin, a peptidolactone antibiotic from Streptomyces-Griseoviridus. Can J Biochem 51(12):1630–1637. https://doi.org/10.1139/o73-219
Houston DR, Eggleston I, Synstad B, Eijsink VGH, van Aalten DMF (2002) The cyclic dipeptide CI-4 cyclo-(L-Arg-D-Pro) inhibits family 18 chitinases by structural mimicry of a reaction intermediate. Biochem J 368:23–27. https://doi.org/10.1042/bj20021034
Huang Y, Zhang WY, Shi Q, Toyo’oka T, Min JZ (2018) Determination of d, l-amino acids in collagen from pig and cod skins by UPLC using pre-column fluorescent derivatization. Food Anal Methods 11(11):3130–3137. https://doi.org/10.1007/s12161-018-1288-9
Huang WJ, Wang YR, Li X, Zhang YL (2020) Biosynthesis and regulation of salicylic acid and N-hydroxypipecolic acid in plant immunity. Mol Plant 13(1):31–41. https://doi.org/10.1016/j.molp.2019.12.008
Hudlický M (1993) Stereospecific syntheses of all 4 stereoisomers of 4-fluoroglutamic acid. J Fluorine Chem 60(2–3):193–210. https://doi.org/10.1016/s0022-1139(00)80034-2
Hušek P, Pohlídal A, Slabík D (2002) Rapid screening of urinary proline-hydroxyproline dipeptide in bone turnover studies. J Chromatogr B Analyt Technol Biomed Life Sci 767(1):169–174. https://doi.org/10.1016/s0378-4347(01)00558-8
Hušek P, Švagera Z, Všianský F, Franeková J, Šimek P (2008) Prolyl-hydroxyproline dipeptide in non-hydrolyzed morning urine and its value in postmenopausal osteoporosis. Clin Chem Lab Med 46(10):1391–1397. https://doi.org/10.1515/cclm.2008.259
Ianni F, Pucciarini L, Carotti A, Sardella R, Natalini B (2019) Enantioseparations by high-performance liquid chromatography based on chiral ligand exchange. Methods Mol Biol 1985:279–302. https://doi.org/10.1007/978-1-4939-9438-0_15
Ilisz I, Tourwe D, Armstrong DW, Peter A (2006) High-performance liquid chromatographic enantioseparation of unusual secondary amino acids on a D-penicillamine-based chiral ligand exchange column. Chirality 18(7):539–543. https://doi.org/10.1002/chir.20257
Ilisz I, Gecse Z, Pataj Z, Fulop F, Toth G, Lindner W, Peter A (2014) Direct high-performance liquid chromatographic enantioseparation of secondary amino acids on Cinchona alkaloid-based chiral zwitterionic stationary phases. Unusual temperature behavior. J Chromatogr A 1363:169–177. https://doi.org/10.1016/j.chroma.2014.06.087
Inoue H, Iguch H, Kouno A, Tsuruta Y (2001) Fluorometric determination of N-terminal prolyl dipeptides, proline and hydroxyproline in human serum by pre-column high-performance liquid chromatography using 4-(5,6-dimethoxy-2-phthalimidinyl)-2-methoxyphenylsulfonyl chloride. J Chromatogr B 757(2):369–373. https://doi.org/10.1016/s0378-4347(01)00162-1
Ishii C, Akita T, Mita M, Ide T, Hamase K (2018) Development of an online two-dimensional high-performance liquid chromatographic system in combination with tandem mass spectrometric detection for enantiomeric analysis of free amino acids in human physiological fluid. J Chromatogr A 1570:91–98. https://doi.org/10.1016/j.chroma.2018.07.076
Jackson PL, Noerager BD, Jablonsky MJ, Hardison MT, Cox BD, Patterson JC, Dhanapal B, Blalock JE, Muccio DD (2011) A CXCL8 receptor antagonist based on the structure of N-acetyl-proline -glycine-proline. Eur J Pharmacol 668(3):435–442. https://doi.org/10.1016/j.ejphar.2011.02.045
Jamal Q, Cho JY, Moon JH, Munir S, Anees M, Kim KY (2017) Identification for the first time of Cyclo(d-Pro-l-Leu) produced by bacillus amyloliquefaciens Y1 as a nematocide for control of meloidogyne incognita. Molecules. https://doi.org/10.3390/molecules22111839
Jin DR, Miyahara T, Oe T, Toyo’oka T (1999) Determination of D-amino acids labeled with fluorescent chiral reagents, R(-)- and S(+)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N, N-dimethylaminosulfonyl)-2,1,3-benzoxadiazoles, in biological and food samples by liquid chromatography. Anal Biochem 269(1):124–132. https://doi.org/10.1006/abio.1998.3090
Johnston GAR, Krogsgaardlarsen P, Stephanson AL, Twitchin B (1976) Inhibition of uptake of GABA and related amino-acids in rat-brain slices by optical isomers of nipecotic acid. J Neurochem 26(5):1029–1032. https://doi.org/10.1111/j.1471-4159.1976.tb06488.x
Jourdant A, Zhu JP (2000) An efficient stereoselective and stereodivergent synthesis of (2R,3R)- and (2R,3S)-3-hydroxypipecolic acids. Tetrahedron Lett 41(36):7033–7036. https://doi.org/10.1016/s0040-4039(00)01207-7
Jurjens G, Schuler SMM, Kurz M, Petit S, Couturier C, Jeannot F, Nguyen F, Wende RC, Hammann PE, Wilson DN, Bacque E, Poverlein C, Bauer A (2018) Total synthesis and structural revision of the antibiotic tetrapeptide GE81112A. Angewandte Chemie-International Edition 57(37):12157–12161. https://doi.org/10.1002/anie.201805901
Kalíková K, Šlechtová T, Tesařová E (2016) Enantiomeric ratio of amino acids as a tool for determination of aging and disease diagnostics by chromatographic measurement. Separations. https://doi.org/10.3390/separations3040030
Kamble RB, Gadre SH, Suryavanshi GM (2015) A formal asymmetric synthesis of (2S,4R)-4-hydroxypipecolic acid via Co(III)(salen)-catalyzed two stereocentered HKR of racemic azido epoxide. Tetrahedron Lett 56(10):1263–1265. https://doi.org/10.1016/j.tetlet.2015.01.113
Kim JS, Kim JC, Lee S, Lee BH, Cho KY (2006) Biological activity of L-2-azetidinecarboxylic acid, isolated from Polygonatum odoratum var. pluriflorum, against several algae. Aquat Bot 85(1):1–6. https://doi.org/10.1016/j.aquabot.2006.01.003
Kim HS, Lee AY, Choi G, Kang YM, Kim HK (2015) Development of ultra-performance liquid chromatography method using hydrophilic interaction liquid chromatography for quantification of azetidine-2-carboxylic acid in rhizomes of polygonatum sibiricum F Delaroche. J Liquid Chromatogr Related Technol 38(16):1515–1520. https://doi.org/10.1080/10826076.2015.1057643
Kimura R, Tsujimura H, Tsuchiya M, Soga S, Ota N, Tanaka A, Kim H (2020) Development of a cognitive function marker based on D-amino acid proportions using new chiral tandem LC-MS/MS systems. Scient Rep. https://doi.org/10.1038/s41598-020-57878-y
Kirschner DL, Green TK (2009) Separation and sensitive detection of D-amino acids in biological matrices. J Sep Sci 32(13):2305–2318. https://doi.org/10.1002/jssc.200900101
Kite GC (1999) Differentiation of epimeric mono- and dihydroxypipecolic acids by negative ion sequential mass spectrometry. Rapid Commun Mass Spectrom 13(11):1063–1066. https://doi.org/10.1002/(sici)1097-0231(19990615)13:11%3c1063::aid-rcm609%3e3.0.co;2-f
Kite GC, Hughes MJ (1997) Analysis of hydroxypipecolic by gas chromatography mass spectrometry. Phytochem Anal 8(6):294–300. https://doi.org/10.1002/(sici)1099-1565(199711/12)8:6%3c294::aid-pca372%3e3.3.co;2-i
Kite GC, Ireland H (2002) Non-protein amino acids of Bocoa (Leguminosae; Papilionoideae). Phytochemistry 59(2):163–168. https://doi.org/10.1016/s0031-9422(01)00447-2
Kite GC, Wieringa JJ (2003) Hydroxypipecolic and hydroxyprolines as chemical characters in Aphanocalyx, Bikinia and Tetraberlinia (Leguminosae : Caesalpinioideae): support for the segregation of Monopetalanthus. Biochem Syst Ecol 31(3):279–292. https://doi.org/10.1016/s0305-1978(02)00152-7
Kodama H, Sugahara K (1997) Analyses of iminodipeptides containing C-terminal proline or hydroxyproline in biological samples by liquid chromatography mass spectrometry. Anal Chim Acta 352(1–3):141–153. https://doi.org/10.1016/s0003-2670(97)00130-x
Koenig S, Marco H, Gade G (2017) The hypertrehalosemic neuropeptides of cicadas are structural isomers-evidence by ion mobility mass spectrometry. Anal Bioanal Chem 409(27):6415–6420. https://doi.org/10.1007/s00216-017-0583-4
Konig S, Marco H, Gade G (2018) D-Proline: Comment to “An overview on D-amino acids.” Amino Acids 50(2):359–361. https://doi.org/10.1007/s00726-017-2511-5
Košťál V, Korbelová J, Rozsypal J, Zahradníčková H, Cimlová J, Tomčala A, Šimek P (2011) Long-term cold acclimation extends survival time at 0 degrees c and modifies the metabolomic profiles of the larvae of the fruit fly Drosophila melanogaster. PLoS ONE. https://doi.org/10.1371/journal.pone.0025025
Košťál V, Šimek P, Zahradníčková H, Cimlová J, Štětina T (2012) Conversion of the chill susceptible fruit fly larva (Drosophila melanogaster) to a freeze tolerant organism. Proc Natl Acad Sci USA 109(9):3270–3274. https://doi.org/10.1073/pnas.1119986109
Kostal V, Korbelova J, Poupardin R, Moos M, Simek P (2016) Arginine and proline applied as food additives stimulate high freeze tolerance in larvae of Drosophila melanogaster. J Exp Biol 219(15):2358–2367. https://doi.org/10.1242/jeb.142158
Krishnakumari V, Sharadadevi A, Singh S, Nagaraj R (2003) Single disulfide and linear analogues corresponding to the carboxy-terminal segment of bovine beta-defensin-2: Effects of introducing the beta-hairpin nucleating sequence D-Pro-Gly on antibacterial activity and biophysical properties. Biochemistry 42(31):9307–9315. https://doi.org/10.1021/bi034403y
Krogsgaardlarsen P, Johnston GAR (1975) Inhibition of GABA uptake in rat brain slices by nipecotic acid, various isoxazoles and related compounds. J Neurochem 25(6):797–802. https://doi.org/10.1111/j.1471-4159.1975.tb04410.x
Kumar SN, Mohandas C (2014) Antimycobacterial activity of cyclic dipeptides isolated from Bacillus sp N strain associated with entomopathogenic nematode. Pharm Biol 52(1):91–96. https://doi.org/10.3109/13880209.2013.815635
Kumar SN, Mohandas C, Siji JV, Rajasekharan KN, Nambisan B (2012) Identification of antimicrobial compound, diketopiperazines, from a Bacillus sp N strain associated with a rhabditid entomopathogenic nematode against major plant pathogenic fungi. J Appl Microbiol 113(4):914–924. https://doi.org/10.1111/j.1365-2672.2012.05385.x
Kumar SN, Nambisan B, Mohandas C (2014a) Purification and identification of two antifungal cyclic dipeptides from Bacillus cereus subsp thuringiensis associated with a rhabditid entomopathogenic nematode especially against Fusarium oxysporum. J Enzyme Inhib Med Chem 29(2):190–197. https://doi.org/10.3109/14756366.2013.765414
Kumar SN, Nambisan B, Sundaresan A, Mohandas C, Anto RJ (2014b) Isolation and identification of antimicrobial secondary metabolites from Bacillus cereus associated with a rhabditid entomopathogenic nematode. Annl Microbiol 64(1):209–218. https://doi.org/10.1007/s13213-013-0653-6
Kunii Y, Otsuka M, Kashino S, Takeuchi H, Ohmori S (1996) 4-Hydroxypipecolic acid and pipecolic acid in Acacia species: Their determination by high-performance liquid chromatography, its application to leguminous plants, and configuration of 4-hydroxypipecolic acid. J Agric Food Chem 44(2):483–487. https://doi.org/10.1021/jf950214d
Langrock T, Garcia-Villar N, Hoffmann R (2007) Analysis of hydroxyproline isomers and hydroxylysine by reversed-phase HPLC and mass spectrometry. J Chromatogr B-Analyt Technol Biomed Life Sci 847(2):282–288. https://doi.org/10.1016/j.jchromb.2006.10.015
Larsson OM, Krogsgaardlarsen P, Schousboe A (1985) Characterization of the uptake of GABA, Nipecotic acid and cis-4-OH-nipecotic acid in cultured neurons and astrocytes. Neurochem Int 7(5):853–860. https://doi.org/10.1016/0197-0186(85)90041-5
Leandro J, Houten SM (2020) The lysine degradation pathway: Subcellular compartmentalization and enzyme deficiencies. Mol Genet Metab 131(1–2):14–22. https://doi.org/10.1016/j.ymgme.2020.07.010
Lee J, Kim KR, Won S, Kim JH, Goto J (2001) Enantioseparation of chiral amino acids as the N(O, S)-ethoxycarbonylated diastereomeric esters by achiral dual-capillary column gas chromatography. Analyst 126(12):2128–2133. https://doi.org/10.1039/b106610m
Leete E (1964) Biosynthesis of azetidine-2-carboxylic acid. J Am Chem Soc 86(15):3162–4000. https://doi.org/10.1021/ja01069a045
Leete E (1975) Biosynthesis of azetidine-2-carboxylic acid from methionine in Nicotiana-Tabacum. Phytochemistry 14(9):1983–1984. https://doi.org/10.1016/0031-9422(75)83109-8
Leete E, Louters LL, Rao HSP (1986) Biosynthesis of azetidine-2-carboxylic acid in Convallaria-Majalis - studies with N-15 labeled precursors. Phytochemistry 25(12):2753–2758. https://doi.org/10.1016/s0031-9422(00)83735-8
Lenci E, Trabocchi A (2019) Occurrence of the d-proline chemotype in enzyme inhibitors. Symmetry-Basel. https://doi.org/10.3390/sym11040558
Lerma J, Herreras O, Herranz AS, Munoz D, Delrio RM (1984) Invivo effects of nipecotic acid on levels of extracellular GABA and Taurine, and hippocampal excitability. Neuropharmacology 23(5):595–598. https://doi.org/10.1016/0028-3908(84)90036-4
Levkin PA, Levkina A, Czesla H, Schurig V (2007) Temperature-induced inversion of the elution order of enantiomers in gas chromatography: N-ethoxycarbonyl propylamides and N-trifluoroacetyl ethyl esters of alpha-amino acids on Chirasil-Val-C-11 and Chirasil-Dex stationary phases. Anal Chem 79(12):4401–4409. https://doi.org/10.1021/ac062064j
Li H, Lee BC, Kim TS, Bae KS, Hong J, Choi SH, Bao B, Jung JH (2008) Bioactive cyclic dipeptides from a marine sponge-associated bacterium, Psychrobacter. Biomol Ther 16(4):356–363. https://doi.org/10.4062/biomolther.2008.16.4.356
Li Z, Xing YP, Guo XJ, Cui Y (2017) Development of an UPLC-MS/MS method for simultaneous quantitation of 11 D-amino acids in different regions of rat brain: Application to a study on the associations of D-amino acid concentration changes and Alzheimer’s disease. J Chromatog B-Analyt Technol Biomed Life Sci 1058:40–46. https://doi.org/10.1016/j.jchromb.2017.05.011
Li D, Liu RY, Singh D, Yuan XY, Kachroo P, Raina R (2020) JMJ14 encoded H3K4 demethylase modulates immune responses by regulating defence gene expression and pipecolic acid levels. New Phytol 225(5):2108–2121. https://doi.org/10.1111/nph.16270
Liscio C, Hopley C (2016) Development of a reference measurement procedure and certified reference material for the determination of hydroxyproline in meat. Food Anal Methods 9(6):1461–1469. https://doi.org/10.1007/s12161-015-0329-x
Liu W, Hancock CN, Fischer JW, Harman M, Phang JM (2015) Proline biosynthesis augments tumor cell growth and aerobic glycolysis: involvement of pyridine nucleotides. Sci Rep. https://doi.org/10.1038/srep17206
Lowe G, Vilaivan T (1997) Amino acids bearing nucleobases for the synthesis of novel peptide nucleic acids. J Chem Soc Perkin Transact 1(4):539–546. https://doi.org/10.1039/a603696a
Lüpke M, Brückner H (1998) Gas chromatographic evaluation of amino acid epimerisation in the course of gelatin manufacturing and processing. Zeitschrift Fur Lebensmittel-Untersuchung Und-Forschung Food Res Technol 206(5):323–328. https://doi.org/10.1007/s002170050266
Ma N, Yang YJ, Liu XW, Li SH, Qin Z, Li JY (2020) Plasma metabonomics and proteomics studies on the anti-thrombosis mechanism of aspirin eugenol ester in rat tail thrombosis model. J Proteomics. https://doi.org/10.1016/j.jprot.2019.103631
MacLean AM, White CE, Fowler JE, Finan TM (2009) Identification of a Hydroxyproline Transport System in the Legume Endosymbiont Sinorhizobium meliloti. Mol Plant Microbe Interact 22(9):1116–1127. https://doi.org/10.1094/mpmi-22-9-1116
Madtes P, Redburn D (1985) Metabolism of H-3 nipecotic acid in the rabbit retina. J Neurochem 44(5):1520–1523
McNaught AD, Wilkinson A (1997) IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Online version (2019-) created by S. J. Chalk. https://doi.org/10.1351/goldbook. Blackwell Scientific Publications, Oxford
Mester L, Szabados L, Mester M, Yadav N (1979) Identification by C-13 NMR spectroscopy of trans-5-hydroxypipecolic acid in the leaves of Xylocarpa, a new inhibitor of platelet-aggregation induced by serotonin. Planta Med 35(4):339–341. https://doi.org/10.1055/s-0028-1097226
Metzner L, Kalbitz J, Brandsch M (2004) Transport of pharmacologically active proline derivatives by the human proton-coupled amino acid transporter hPAT1. J Pharmacol Exp Ther 309(1):28–35. https://doi.org/10.1124/jpet.103.059014
Meyer JJM, Grobbelaar N (1986) Biosynthesis of pipecolic acid and 4-hydroxy pipecolic acid. Phytochemistry 25(6):1469–1470. https://doi.org/10.1016/s0031-9422(00)81310-2
Min JZ, Hatanaka S, Yu H, Higashi T, Inagaki S, Toyo’oka T (2011) Determination of DL-amino acids, derivatized with R(-)-4-(3-isothiocyanatopyrrolidin-1-yl)-7-(N, N-dimethylaminosulfonyl)-2,1,3-benzoxadiazole, in nail of diabetic patients by UPLC-ESI-TOF-MS. J Chromatogr B-Anall Technol Biomed Life Sci 879(29):3220–3228. https://doi.org/10.1016/j.jchromb.2011.02.016
Mishra UK, Ramesh NG (2020) A carbohydrate based straightforward approach to trans-4-hydroxy-D-proline and trans-4-hydroxy-D-prolinol. Tetrahedron Lett. https://doi.org/10.1016/j.tetlet.2020.152081
Miyata T, Okano Y, Nagatatanoue J, Ijimamiyamura S, Iwamura H, Takahama K, Hitoshi T (1987) Identification and quantification of 5-hydroxy pipecolic acid and 4-hydroxyproline in mammalian brain and blood by selected ion monitoring. Anal Biochem 163(2):303–308. https://doi.org/10.1016/0003-2697(87)90228-4
Miyazawa T, Minowa H, Imagawa K, Yamada T (1997) Enantiomeric separation of non-protein amino acids by chiral ligand-exchange high-performance liquid chromatography. Anal Lett 30(4):867–882. https://doi.org/10.1080/00032719708006430
Miyazawa T, Minowa H, Imagawa K, Yamada T (2004) Separation of enantiomers of non-protein amino acids by high-performance liquid chromatography on a chiral ligand-exchange column. Chromatographia 60(1–2):45–50. https://doi.org/10.1365/s10337-004-0298-5
Mochizuki T, Takayama T, Todoroki K, Inoue K, Min JZ, Toyo’oka T (2015) Towards the chiral metabolomics: Liquid chromatography-mass spectrometry based DL-amino acid analysis after labeling with a new chiral reagent, (S)-2,5-dioxopyrrolidin-1-yl-1-(4,6-dimethoxy-1,3,5-triazin-2-yl)pyrroli dine-2-carboxylate, and the application to saliva of healthy volunteers. Anal Chim Acta 875:73–82. https://doi.org/10.1016/j.aca.2015.02.054
Moon SJ, Han CT (1998) Proline analogs, L-azetidine-2-carboxylic acid and 3,4-dehydro-L-proline, induce stress response in Drosophila Kc cells. J Biochem Mol Biol 31(2):201–208
More SH, Ganesh KN (2019) Speigelmeric 4R/S-hydroxy/amino-L/D-prolyl collagen peptides: conformation and morphology of self-assembled structures. Pept Sci. https://doi.org/10.1002/pep2.24140
Morgan TD, Chippendale GM (1983) Free amino acids of the hemolymph of the southwestern corn-borer and the european corn-borer in relation to their diapause. J Insect Physiol 29(10):735–740. https://doi.org/10.1016/0022-1910(83)90001-x
Moriuchi T, Nishiyama T, Tayano Y, Hirao T (2017) Controlled self-assembling structures of ferrocene-dipeptide conjugates composed of Ala-Pro-NHCH2CH2SH chain. J Inorg Biochem 177:259–265. https://doi.org/10.1016/j.jinorgbio.2017.05.009
Morton TC (1998) Chemotaxonomic significance of hydroxylated pipecolic acids in Central American Inga (Fabaceae: Mimosoideae: Ingeae). Biochem Syst Ecol 26(4):379–401. https://doi.org/10.1016/s0305-1978(98)00003-9
Nagana Gowda GA, Gowda YN, Raftery D (2015) Massive glutamine cyclization to pyroglutamic acid in human serum discovered using NMR spectroscopy. Anal Chem 87(7):3800–3805. https://doi.org/10.1021/ac504435b
Nagata Y, Masui R, Akino T (1992a) The presence of free D-serine, D-alanine and D-proline in human plasma. Experientia 48(10):986–988. https://doi.org/10.1007/bf01919147
Nagata Y, Yamamoto K, Shimojo T (1992b) Determination of D-amino and L-amino-acids in mouse kidney by high performance liquid-chromatography. J Chromatogr Biomed Appl 575(1):147–152. https://doi.org/10.1016/0378-4347(92)80516-s
Nagata Y, Higashi M, Ishii Y, Sano H, Tanigawa M, Nagata K, Noguchi K, Urade M (2006) The presence of high concentrations of free D-amino acids in human saliva. Life Sci 78(15):1677–1681. https://doi.org/10.1016/j.lfs.2005.08.009
Nakanishi T, Shimizu A, Saiki K, Fujiwara F, Funahashi S, Hayashi A (1991) Quantitative-analysis of urinary pyroglutamic acid in patients with hyperammonemia. Clin Chim Acta 197(3):249–256
Naresh G, Jaiswal N, Sukanya P, Srivastava AK, Tamrakar AK, Narender T (2012) Glucose uptake stimulatory effect of 4-hydroxypipecolic acid by increased GLUT 4 translocation in skeletal muscle cells. Bioorg Med Chem Lett 22(17):5648–5651. https://doi.org/10.1016/j.bmcl.2012.06.101
Nassereddinesebaei M, Crider AM, Carroll RT, Hinko CN (1993) Determination of m-nitrophenol and nipecotic acid in mouse tissues by high performance liquid chromatography after administration of the anticonvulsant m-nitrophenyl-3-piperidinecarboxylate hydrochloride. J Pharm Sci 82(1):39–43. https://doi.org/10.1002/jps.2600820109
Natalini B, Giacche N, Sardella R, Ianni F, Macchiarulo A, Pellicciari R (2010) Computational studies for the elucidation of the enantiomer elution order of amino acids in chiral ligand-exchange chromatography. J Chromatogr A 1217(48):7523–7527. https://doi.org/10.1016/j.chroma.2010.10.001
Navarova H, Bernsdorff F, Doring AC, Zeier J (2012) Pipecolic acid , an endogenous mediator of defense amplification and priming, is a critical regulator of inducible plant immunity. Plant Cell 24(12):5123–5141. https://doi.org/10.1105/tpc.112.103564
Nishizawa N, Niida A, Adachi Y, Kanematsu-Yamaki Y, Masuda Y, Kumano S, Yokoyama K, Noguchi Y, Asakawa T, Hirabayashi H, Amano N, Takekawa S, Ohtaki T, Asami T (2017) Highly potent antiobesity effect of a short-length peptide YY analog in mice. Bioorg Med Chem 25(20):5718–5725. https://doi.org/10.1016/j.bmc.2017.08.044
Nokihara K, Gerhardt J (2001) Development of an improved automated gas-chromatographic chiral analysis system: application to non-natural amino acids and natural protein hydrolysates. Chirality 13(8):431–434. https://doi.org/10.1002/chir.1057
Occhiato EG, Scarpi D, Guarna A, Tabasso S, Deagostino A, Prandi C (2009) A short and convenient synthesis of enantiopure cis- and trans-4-hydroxypipecolic acid. Synthesis-Stuttgart 21:3611–3616. https://doi.org/10.1055/s-0029-1216979
O’Farrell CM, Chudomel JM, Collins JM, Dignam CF, Wenzel TJ (2008) Water-soluble calix 4 resorcinarenes with hydroxyproline groups as chiral NMR solvating agents. J Org Chem 73(7):2843–2851. https://doi.org/10.1021/jo702751z
Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M (1999) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 27(1):29–34. https://doi.org/10.1093/nar/27.1.29
Ohara C, Takahashi R, Miyagawa T, Yoshimura Y, Kato A, Adachi I, Takahata H (2008) Synthesis of all stereoisomers of 3-hydroxypipecolic acid and 3-hydroxy-4,5-dehydro pipecolic acid and their evaluation as glycosidase inhibitors. Bioorg Med Chem Lett 18(6):1810–1813. https://doi.org/10.1016/j.bmcl.2008.02.028
Oi N, Kitahara H, Aoki F (1993) Enantiomer separation by HPLC on reversed phase silica-gel coated with copper(Ii) complexes of (R, R)-tartaric acid mono-amide derivatives. J Liq Chromatogr 16(4):893–901
OkotKotber BM, Adeyeye OA (1997) Changes in catecholamine levels in the gut and frass of the corn earworm, Helicoverpa zea induced by dietary L-azetidine-2-carboxylic acid. Insect Biochem Mol Biol 27(5):431–438. https://doi.org/10.1016/s0965-1748(97)00015-5
Okumura Y, Ohnishi M, Okamoto R, Ishikura T (1982) Some effectors on the biosynthesis of cis-4-hydroxy-D-proline in Viridogrisein. Agric Biol Chem 46(12):3063–3068. https://doi.org/10.1080/00021369.1982.10865545
Opekar S, Zahradníčková H, Vodrážka P, Řimnáčová L, Šimek P, Moos M (2021) A chiral GC-MS method for analysis of secondary amino acids after heptafluorobutyl chloroformate & methylamine derivatization. Amino Acids 53(3):347–358
Padrosa DR, Benitez-Mateos AI, Calvey L, Paradisi F (2020) Cell-free biocatalytic syntheses ofl-pipecolic acid: a dual strategy approach and process intensification in flow. Green Chem 22(16):5310–5316. https://doi.org/10.1039/d0gc01817a
Paik MJ, Lee J, Kim K-R (2008) N-Ethoxycarbonylation combined with (S)-1-phenylethylamidation for enantioseparation of amino acids by achiral gas chromatography and gas chromatography-mass spectrometry. J Chromatogr A 1214(1–2):151–156. https://doi.org/10.1016/j.chroma.2008.10.068
Patzold R, Brückner H (2005) Mass spectrometric detection and formation of D-amino acids in processed plant saps, syrups, and fruit juice concentrates. J Agric Food Chem 53(25):9722–9729. https://doi.org/10.1021/jf051433u
Patzold R, Brückner H (2006) Gas chromatographic detection of D-amino acids in natural and thermally treated bee honeys and studies on the mechanism of their formation as result of the Maillard reaction. Eur Food Res Technol 223(3):347–354. https://doi.org/10.1007/s00217-005-0211-y
Patzold R, Nieto-Rodriquez A, Bruckner H (2003) Chiral gas chromatographic analysis of amino acids in fortified wines. Chromatographia 57:S207–S212. https://doi.org/10.1007/bf02492104
Pawlowska M, Chen SS, Armstrong DW (1993) Enantiomeric separation of fluorescent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, tagged amino-acids. J Chromatogr 641(2):257–265. https://doi.org/10.1016/0021-9673(93)80142-u
Perez-Miguez R, Marina ML, Castro-Puyana M (2016) Enantiomeric separation of non-protein amino acids by electrokinetic chromatography. J Chromatogr A 1467:409–416. https://doi.org/10.1016/j.chroma.2016.06.058
Peter A, Vekes E, Armstrong DW, Tourwe D (2002a) Enantioseparation by HPLC of imino acids on macrocyclic glycopeptide stationary phases and as their (S)-N-(4-nitrophenoxycarbonyl)phenylaianine methoxyethyl ester derivatives. Chromatographia 56:S41–S47
Peter A, Vekes E, Toth G, Tourwe D, Borremans F (2002b) Application of a new chiral derivatizing agent to the enantioseparation of secondary amino acids. J Chromatogr A 948(1–2):283–294. https://doi.org/10.1016/s0021-9673(01)01475-3
Peter A, Torok R, Armstrong DW (2004) Direct high-performance liquid chromatographic separation of unusual secondary amino acids and a comparison of the performances of Chirobiotic T and TAG columns. J Chromatogr A 1057(1–2):229–235. https://doi.org/10.1016/j.chroma.2004.09.027
Peterson PJ, Fowden L (1963) Different specificites of proline -activating enzymes from some plant species. Nature 200(490):148. https://doi.org/10.1038/200148a0
Peterson PJ, Fowden L (1965) Purification properties and comparative specificities of enzyme prolyl-transfer ribonucleic acid synthetase from Phaseolus Aureus and Polygonatum Multiflorum. Biochem J 97(1):112–120. https://doi.org/10.1042/bj0970112
Phang JM (2017) Proline metabolism in cell regulation and cancer biology: recent advances and hypotheses. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7350
Phang JM, Liu W (2012) Proline metabolism and cancer. Front Biosci Landmark 17:1835–1845. https://doi.org/10.2741/4022
Prasad BB, Pandey I (2013) Metal incorporated molecularly imprinted polymer-based electrochemical sensor for enantio-selective analysis of pyroglutamic acid isomers. Sens Actuators B Chem 186:407–416. https://doi.org/10.1016/j.snb.2013.06.041
Pujals S, Sabido E, Tarrago T, Giralt E (2007)all-D proline -rich cell-penetrating peptides: a preliminary in vivo internalization study. Biochem Soc Trans 35:794–796. https://doi.org/10.1042/bst0350794
Purwaha P, Silva LP, Hawke DH, Weinstein JN, Lorenzi PL (2014) An artifact in LC-MS/MS measurement of glutamine and glutamic acid: in-source cyclization to pyroglutamic acid. Anal Chem 86(12):5633–5637. https://doi.org/10.1021/ac501451v
Qiu B, Wei FX, Sun XZ, Wang X, Duan BH, Shi CL, Zhang JY, Zhang JY, Qiu WL, Mu WL (2014) Measurement of hydroxyproline in collagen with three different methods. Mol Med Rep 10(2):1157–1163. https://doi.org/10.3892/mmr.2014.2267
Rao IN, Boruah A, Kumar SK, Kunwar AC, Devi AS, Vyas K, Ravikumar K, Iqbal J (2004) Synthesis and conformational studies of novel cyclic peptides constrained into a 3(10) helical structure by a heterochiral D-pro-L-pro dipeptide template. J Org Chem 69(6):2181–2184. https://doi.org/10.1021/jo030282w
Rashed MS, Al-Ahaidib LY, Aboul-Enein HY, Al-Amoudi M, Jacob M (2001) Determination of L-pipecolic acid in plasma using chiral liquid chromatography-electrospray tandem mass spectrometry. Clin Chem 47(12):2124–2130
Ren Y, Zhao JJ, Shi YN, Chen CY, Chen XM, Lv C (2017) Simple determination of L-hydroxyproline in idiopathic pulmonary fibrosis lung tissues of rats using non-extractive high-performance liquid chromatography coupled with fluorescence detection after pre-column derivatization with novel synthetic 9-acetylimidazol-carbazole. J Pharm Biomed Anal 142:1–6. https://doi.org/10.1016/j.jpba.2017.04.033
Robinson JA (2008) Beta-hairpin peptidomimetics: design, structures and biological activities. Acc Chem Res 41(10):1278–1288. https://doi.org/10.1021/ar700259k
Romeo JT (1984) Insecticidal imino acids in leaves of Calliandra. Biochem Syst Ecol 12(3):293–297. https://doi.org/10.1016/0305-1978(84)90052-8
Romeo JT, Swain LA, Bleecker AB (1983) Cis-4-hydroxypipecolic acid and 2,4-cis-4,5-trans-4,5-dihydroxy pipecolic acid from Calliandra. Phytochemistry 22(7):1615–1617. https://doi.org/10.1016/0031-9422(83)80098-3
Rosenthal GA (1998) The effect of nonprotein amino acid administration on the lysozyme activity of the tobacco hornworm, Manduca sexta Sphingidae. Biochem Syst Ecol 26(3):257–266. https://doi.org/10.1016/s0305-1978(97)00100-2
Rubenstein E (2013) Azetidine-2-carboxylic acid and other nonprotein amino acids in the pathogenesis of neurodevelopmental disorders. Compr Dev Neurosci. https://doi.org/10.1016/b978-0-12-397267-5.00220-x
Rubenstein E, Zhou HL, Krasinska KM, Chien A, Becker CH (2006) Azetidine-2-carboxylic acid in garden beets (Beta vulgaris). Phytochemistry 67(9):898–903. https://doi.org/10.1016/j.phytochem.2006.01.028
Rubenstein E, McLaughlin T, Winant RC, Sanchez A, Eckart M, Krasinska KM, Chien A (2009) Azetidine-2-carboxylic acid in the food chain. Phytochemistry 70(1):100–104. https://doi.org/10.1016/j.phytochem.2008.11.007
Samardzic K, Rodgers KJ (2019) Cell death and mitochondrial dysfunction induced by the dietary non-proteinogenic amino acid l-azetidine-2-carboxylic acid (Aze). Amino Acids 51(8):1221–1232. https://doi.org/10.1007/s00726-019-02763-w
Sanaie N, Haynes CA (2006) Interpreting the effects of temperature and solvent composition on separation of amino-acid racemates by chiral ligand-exchange chromatography. J Chromatogr A 1104(1–2):164–172. https://doi.org/10.1016/j.chroma.2005.11.111
Sardella R, Ianni F, Natalini B, P. Blanch G, L. R. del Castillo M, (2012a) Rapid detection of D-amino acids in cheese with a chiral ligand- exchange chromatography system. Curr Anal Chem 8(2):319–327. https://doi.org/10.2174/157341112800392562
Sardella R, Macchiarulo A, Carotti A, Ianni F, Rubino ME, Natalini B (2012b) Chiral mobile phase in ligand-exchange chromatography of amino acids: exploring the copper(II) salt anion effect with a computational approach. J Chromatogr A 1269:316–324. https://doi.org/10.1016/j.chroma.2012.08.018
Sardella R, Ianni F, Lisanti A, Scorzoni S, Marinozzi M, Natalini B (2015) S-Trityl-(R)-cysteine, a multipurpose chiral selector for ligand-exchange liquid chromatography applications. Crit Rev Anal Chem 45(4):323–333
Sato T, Hirayama F, Saito T, Kaniwa H (1991) A new alkaloid antibiotic tetrazomine structure determination. J Antibiot 44(12):1367–1370
Sato T, Hoshida H, Akada R (2020) Inhibition of distinct proline - or N-acetylglucosamine-induced hyphal formation pathways by proline analogs in candida albicans. Biomed Res Int. https://doi.org/10.1155/2020/7245782
Schieber A, Brückner H, RuppClassen M, Specht W, NowitzkiGrimm S, Classen HG (1997) Evaluation of D-amino acid levels in rat by gas chromatography-selected ion monitoring mass spectrometry: No evidence for subacute toxicity of orally fed D-proline and D-aspartic acid. J Chromatogr B 691(1):1–12. https://doi.org/10.1016/s0378-4347(96)00378-7
Schmidt SK, Hofner G, Wanner KT (2017) Determination of enantiomeric excess of nipecotic acid as 1-(7-nitrobenzo c 1,2,5 oxadiazol-4-yl) derivatives. Chirality 29(1):48–56. https://doi.org/10.1002/chir.22670
Scott JD, Tippie TN, Williams RM (1998) Synthetic studies on tetrazomine: Stereochemical assignment of the beta-hydroxypipecolic acid. Tetrahedron Lett 39(22):3659–3662. https://doi.org/10.1016/s0040-4039(98)00642-x
Shankaramma SC, Athanassiou Z, Zerbe O, Moehle K, Mouton C, Bernardini F, Wrijbloed JW, Obrecht D, Robinson JA (2002) Macrocyclic hairpin mimetics of the cationic antimicrobial peptide protegrin I: A new family of broad-spectrum antibiotics. ChemBioChem 3(11):1126–1133. https://doi.org/10.1002/1439-7633(20021104)3:11%3c1126::aid-cbic1126%3e3.0.co;2-i
Sharma S, Choudhary B, Yadav S, Mishra A, Mishra VK, Chand R, Chen C, Pandey SP (2021) Metabolite profiling identified pipecolic acid as an important component of peanut seed resistance against Aspergillus flavus infection. J Hazard Mater. https://doi.org/10.1016/j.jhazmat.2020.124155
Shigemura A, Chowdhury VS, Furuse M (2020) Intracerebroventricular injection of L-pipecolic acid exerts hypnotic effects without activating NMDA receptors in neonatal chicks under social isolation-induced stress. J Poultry Sci 57(1):84–87. https://doi.org/10.2141/jpsa.0190067
Shiota R, Morita H, Matsumoto T, Morimoto A, Hayakawa J, Oka M, Kamimori H (2017) Bioanalytical method for the determination of hydroxyproline in mouse kidney by high-performance liquid chromatography with tandem mass spectrometric detection. Anal Sci 33(6):719–722. https://doi.org/10.2116/analsci.33.719
Singh AB, Khaliq T, Chaturvedi JP, Narender T, Srivastava AK (2012) Anti-diabetic and anti-oxidative effects of 4-hydroxypipecolic acid in C57BL/KsJ-db/db mice. Hum Exp Toxicol 31(1):57–65. https://doi.org/10.1177/0960327111407227
Son S, Ko S-K, Jang M, Lee JK, Ryoo I-J, Lee J-S, Lee KH, Soung N-K, Oh H, Hong Y-S, Kim BY, Jang J-H, Ahn JS (2015) Ulleungamides A and B, modified alpha, beta-dehydropipecolic acid containing cyclic depsipeptides from streptomyces sp KCB13F003. Org Lett 17(16):4046–4049. https://doi.org/10.1021/acs.orglett.5b01969
Sotelo CG, Comesana MB, Ariza PR, Perez-Martin RI (2016) Characterization of collagen from different discarded fish species of the west coast of the Iberian peninsula. J Aquat Food Prod Technol 25(3):388–399. https://doi.org/10.1080/10498850.2013.865283
Soudijn W, van Wijngaarden I (2000) The GABA transporter and its inhibitors. Curr Med Chem 7(10):1063–1079. https://doi.org/10.2174/0929867003374363
Spanik I, Horvathova G, Janacova A, Krupcik J (2007) On the use of solid phase ion exchangers for isolation of amino acids from liquid samples and their enantioselective gas chromatographic analysis. J Chromatogr A 1150(1–2):145–154. https://doi.org/10.1016/j.chroma.2007.01.070
Spath J, Stuart F, Jiang LY, Robinson JA (1998) Stabilization of a beta-hairpin conformation in a cyclic peptide using the templating effect of a heterochiral diproline unit. Helv Chim Acta 81(9):1726–1738. https://doi.org/10.1002/(sici)1522-2675(19980909)81:9%3c1726::aid-hlca1726%3e3.0.co;2-h
Srivastava AK, Khare P, Nagar HK, Raghuwanshi N, Srivastava R (2016) Hydroxy proline: a potential biochemical marker and its role in the pathogenesis of different diseases. Curr Protein Pept Sci 17(6):596–602. https://doi.org/10.2174/1389203717666151201192247
Staden R, Nejem RM, van Staden JF, Aboul-Enein HY (2008) Sequential injection analysis utilizing amperometric biosensors as detectors for the simultaneous determination of L- and D-pipecolic acid. Instrum Sci Technol 36(4):355–366. https://doi.org/10.1080/10739140802151366
Stahl E, Hartmann M, Scholten N, Zeier J (2019) A Role for tocopherol biosynthesis in arabidopsis basal immunity to bacterial infection. Plant Physiol 181(3):1008–1028. https://doi.org/10.1104/pp.19.00618
Stefan RI, Nejem RM, van Staden JF, Aboul-Enein HY (2004) New amperometric biosensors based on diamond paste for the assay of L- and D-pipecolic acids in serum samples. Prep Biochem Biotechnol 34(2):135–143. https://doi.org/10.1081/pb-120030872
Stefan-van Staden RI, Moldoveanu I, Sava DF, Kapnissi-Christodoulou C, Van Staden JF (2013) Enantioanalysis of pipecolic acid with stochastic and potentiometric microsensors. Chirality 25(2):114–118. https://doi.org/10.1002/chir.22119
Stringham RW (2005) Chiral separation of amines in subcritical fluid chromatography using polysaccharide stationary phases and acidic additives. J Chromatogr A 1070(1–2):163–170
Struys EA, Jakobs C (1999) Enantiomeric analysis of D- and L-pipecolic acid in plasma using a chiral capillary gas chromatography column and mass fragmentography-. J Inherit Metab Dis 22(5):677–678. https://doi.org/10.1023/a:1005558903769
Sun TJ, Huang JH, Xu Y, Verma V, Jing BB, Sun YL, Orduna AR, Tian HN, Huang XC, Xia ST, Schafer L, Jetter R, Zhang YL, Li X (2020) Redundant CAMTA transcription factors negatively regulate the biosynthesis of salicylic acid and N-hydroxypipecolic acid by modulating the expression of SARD1 and CBP60g. Mol Plant 13(1):144–156. https://doi.org/10.1016/j.molp.2019.10.016
Sung ML, Fowden L (1968) Trans-3-hydroxy-L-proline - a constituent of delonix regia. Phytochemistry 7(11):2061–3000. https://doi.org/10.1016/s0031-9422(00)90769-6
Takagi T, Ando R, Ohgushi A, Yamashita T, Dobashi E, Hussain-Yusuf H, Onodera R, Bungo T, Sato H, Furuse M (2001) Intracerebroventricular injection of pipecolic acid inhibits food intake and induces sleeping-like behaviors in the neonatal chick. Neurosci Lett 310(2–3):97–100. https://doi.org/10.1016/s0304-3940(01)02059-6
Takagi T, Bungo T, Tachibana T, Saito ES, Saito S, Yamasaki I, Tomonaga S, Denbow DM, Furuse M (2003) Intracerebroventricular administration of GABA-A and GABA-B receptor antagonists attenuate feeding and sleeping-like behavior induced by L-pipecolic acid in neonatal chicks. J Neurosci Res 73(2):270–275. https://doi.org/10.1002/jnr.10656
Takahashi KI, Miyoshi S, Kaneko A, Copenhagen DR (1995) Actions of nipecotic acid and SKFf89976a on GABA transporter in cone-driven horizontal cells dissociated from the catfish retina Japanese J Physiol 45:457–473
Takeuchi T, Prockop DJ (1969) Biosynthesis of abnormal collagens with amino acid analogues. I .Incorporation of L-Azetidine-2-carboxylic acid and cis-4-fluoro-L-proline into protocollagen and collagen. Biochem Biophys Acta 175(1):142. https://doi.org/10.1016/0005-2795(69)90153-6
Tanaka N, Suto S, Ishiyama H, Kubota T, Yamano A, Shiro M, Fromont J, Kobayashi J (2012) Halichonadins K and L, New Dimeric Sesquiterpenoids from a Sponge Halichondria sp. Org Lett 14(13):3498–3501. https://doi.org/10.1021/ol3014705
Taylor CM, Hardre R, Edwards PJB (2005) The impact of pyrrolidine hydroxylation on the conformation of proline-containing peptides. J Org Chem 70(4):1306–1315.https://doi.org/10.1021/jo0490043
Teng ZY, Lv HY, Zhang YY, Zhang Y, Guan J, Wang CY (2016) Development of a fluorescent labeling reagent for determination of proline and hydroxyproline in myeloma patient plasma by HPLC-FLD. J Chromatogr Sci 54(10):1743–1751. https://doi.org/10.1093/chromsci/bmw132
Thompson JF, Morris CJ (1968) Conversion of 5-hydroxylysine to 5-hydroxypipecolic acid in honey locust leaves. Arch Biochem Biophys 125(1):362–370. https://doi.org/10.1016/0003-9861(68)90671-1
Tojo Y, Hamase K, Nakata M, Morikawa A, Mita M, Ashida Y, Lindner W, Zaitsu K (2008) Automated and simultaneous two-dimensional micro-high-performance liquid chromatographic determination of proline and hydroxy proline enantiomers in mammals. J Chromatogra B Anal Technol Biomed Life Sci 875(1):174–179. https://doi.org/10.1016/j.jchromb.2008.06.025
Tojo M, Murakami M, Nagata Y (2012) Simple and low-cost high-performance liquid chromatographic method for determination of D- and L-amino acids. J Chromatogr Sci 50(5):393–395. https://doi.org/10.1093/chromsci/bmr004
Twardzik DR, Peterkofsky A (1972) Glutamic-acid as a precursor to n-terminal pyroglutamic acid in mouse plasmacytoma protein. Proce Natl Acad Sci USA 69(1):274
van Rijn J, van den Berg J, van der Mast CA (1999) Effects of azetidine-2-carboxylic acid on treatments of hepatoma cells with single or fractionated X-ray irradiations and on thermal radiosensitization in normal and thermotolerant cells. Radiat Oncol Investig 7(5):270–277. https://doi.org/10.1002/(sici)1520-6823(1999)7:5%3c270::aid-roi2%3e3.0.co;2-u
Vanlerberghe GC, Brown LM (1987) Proline overproduction in cells of the green-alga nannochloris-bacillaris resistant to azetidine-2-carboxylic acid. Plant Cell Environ 10(3):251–257
Velíšek J, Kubec R, Cejpek K (2006) Biosynthesis of food constituents: Amino acids: 4. Non-protein amino acids - A review. Czech J Food Sci 24 (3):93–109. https://doi.org/10.17221/3304-cjfs
Visser WF, Verhoeven-Duif NM, Ophoff R, Bakker S, Klomp LW, Berger R, de Koning TJ (2011) A sensitive and simple ultra-high-performance-liquid chromatography-tandem mass spectrometry based method for the quantification of D-amino acids in body fluids. J Chromatogr A 1218(40):7130–7136. https://doi.org/10.1016/j.chroma.2011.07.087
Vránová V, Lojková L, Rejsek K, Formánek P (2013) Significance of the natural occurrence of l- versus d-pipecolic acid: a review. Chirality 25(12):823–831. https://doi.org/10.1002/chir.22237
Wang HN, Hussain AA, Pyrek JS, Goodman J, Wedlund PJ (2004) Assay for nipecotic acid in small blood samples by gas chromatography-mass spectroscopy. J Pharm Biomed Anal 34(5):1063–1070. https://doi.org/10.1016/j.jpba.2003.11.019
Warren CR, Aranda I, Cano FJ (2011) Responses to water stress of gas exchange and metabolites in Eucalyptus and Acacia spp. Plant Cell Environ 34(10):1609–1629. https://doi.org/10.1111/j.1365-3040.2011.02357.x
Watanabe LA, Haranaka S, Jose B, Yoshida M, Kato T, Moriguchi M, Soda K, Nishino N (2005) An efficient access to both enantiomers of pipecolic acid. Tetrahedron Asymmetry 16(4):903–908. https://doi.org/10.1016/j.tetasy.2005.01.017
Wattana-Amorn P, Charoenwongsa W, Williams C, Crump MP, Apichaisataienchote B (2016) Antibacterial activity of cyclo(L-Pro-L-Tyr) and cyclo(D-Pro-L-Tyr) from Streptomyces sp. strain 22–4 against phytopathogenic bacteria. Natl Prod Res 30(17):1980–1983. https://doi.org/10.1080/14786419.2015.1095747
Wegener S, Kaufmann M, Kroh LW (2017) Influence of l-pyroglutamic acid on the color formation process of non-enzymatic browning reactions. Food Chem 232:450–454. https://doi.org/10.1016/j.foodchem.2017.04.046
Wen J, Liao H, Stachowski K, Hempfling JP, Qian ZQ, Yuan CH, Foster MP, Pei DH (2020) Rational design of cell-permeable cyclic peptides containing a D-Pro-L-Pro motif. Bioorgan Med Chem. https://doi.org/10.1016/j.bmc.2020.115711
Wijdeven MA, Willemsen J, Rutjes F (2010) The 3-hydroxypiperidine skeleton: key element in natural product synthesis. Eur J Org Chem 15:2831–2844. https://doi.org/10.1002/ejoc.200901494
Wishart DS, Feunang YD, Marcu A, Guo AC, Liang K, Vazquez-Fresno R, Sajed T, Johnson D, Li CR, Karu N, Sayeeda Z, Lo E, Assempour N, Berjanskii M, Singhal S, Arndt D, Liang YJ, Badran H, Grant J, Serra-Cayuela A, Liu YF, Mandal R, Neveu V, Pon A, Knox C, Wilson M, Manach C, Scalbert A (2018) HMDB 4.0: the human metabolome database for 2018. Nucleic Acids Res 46(D1):D608–D617
Witkop B, Foltz CM (1957) The configuration of 5-hydroxypipecolic acid from dates. J Am Chem Soc 79(1):192–197. https://doi.org/10.1021/ja01558a051
Wu GY, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li XL, McKnight JR, Satterfield MC, Spencer TE (2011) Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino Acids 40(4):1053–1063. https://doi.org/10.1007/s00726-010-0715-z
Wu ZL, Hou YQ, Dai ZL, Hu CAA, Wu GY (2017) Metabolism, nutrition, and redox signaling of hydroxyproline. Antioxid Redox Signal. https://doi.org/10.1089/ars.2017.7338
Ye YK, Lord BS, Yin L, Stringham RW (2002) Enantioseparation of amino acids on a polysaccharide-based chiral stationary phase. J Chromatogr A 945(1–2):147–159
Yeung KF, Lee KM, Woodard RW (1998) Isolation and identification of two L-azetidine-2-carboxylic acid-degrading soil microorganisms, Enterobacter agglomerans and Enterobacter amnigenus. J Nat Prod 61(2):207–211. https://doi.org/10.1021/np970324+
Yokoyama T, Tokuda M, Amano M, Mikami K (2017) Simultaneous determination of primary and secondary d- and l-amino acids by reversed-phase high-performance liquid chromatography using pre-column derivatization with two-step labelling method. Biosci Biotechnol Biochem 81(9):1681–1686. https://doi.org/10.1080/09168451.2017.1340090
Zhang N, Zhu QF, Gong MJ (2017) Rapid determination of free prolyl dipeptides and 4-hydroxyproline in urine using flow-gated capillary electrophoresis. Anal Bioanal Chem 409(30):7077–7085. https://doi.org/10.1007/s00216-017-0666-2
Zielinska M, Chen CQ, Mokrowiecka A, Cygankiewicz AI, Zakrzewski PK, Salaga M, Malecka-Panas E, Wlaz P, Krajewska WM, Fichna J (2015) Orally administered novel cyclic pentapeptide P-317 alleviates symptoms of diarrhoea-predominant irritable bowel syndrome. J Pharm Pharmacol 67(2):244–254. https://doi.org/10.1111/jphp.12335
Zukowski J, Pawlowska M, Armstrong DW (1992) Efficient enantioselective separation and determination of trace impurities in secondary amino-acids (ie, imino acids). J Chromatogr 623(1):33–41. https://doi.org/10.1016/0021-9673(92)85295-5
Acknowledgements
The authors acknowledge the financial support of the ERDF Fund, the Interreg project No. BYCZ118.
Author information
Authors and Affiliations
Contributions
HZ, SO, and MM prepared the manuscript. LŘ and PŠ conducted an internal review procedure. All authors approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethics approval
No human or animal samples were used for this study. For this reason, no informed consent was obtained from individual participants.
Additional information
Handling editor: N. Vanthuyne.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zahradníčková, H., Opekar, S., Řimnáčová, L. et al. Chiral secondary amino acids, their importance, and methods of analysis. Amino Acids 54, 687–719 (2022). https://doi.org/10.1007/s00726-022-03136-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-022-03136-6