Abstract
Schizophrenia is a severe psychiatric disorder of neurodevelopmental origin that affects around 1% of the world’s population. Proteomic studies and other approaches have provided evidence of compromised cellular processes in the disorder, including mitochondrial function. Most of the studies so far have been conducted on postmortem brain tissue from patients, and therefore, do not allow the evaluation of the neurodevelopmental aspect of the disorder. To circumvent that, we studied the mitochondrial and nuclear proteomes of neural stem cells (NSCs) and neurons derived from induced pluripotent stem cells (iPSCs) from schizophrenia patients versus healthy controls to assess possible alterations related to energy metabolism and mitochondrial function during neurodevelopment in the disorder. Our results revealed differentially expressed proteins in pathways related to mitochondrial function, cell cycle control, DNA repair and neuritogenesis and their possible implication in key process of neurodevelopment, such as neuronal differentiation and axonal guidance signaling. Moreover, functional analysis of NSCs revealed alterations in mitochondrial oxygen consumption in schizophrenia-derived cells and a tendency of higher levels of intracellular reactive oxygen species (ROS). Hence, this study shows evidence that alterations in important cellular processes are present during neurodevelopment and could be involved with the establishment of schizophrenia, as well as the phenotypic traits observed in adult patients.
Graphical abstract
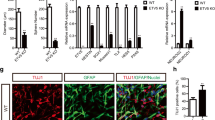
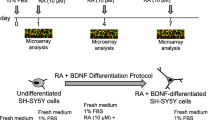
Neural stem cells (NSCs) and neurons were derived from induced pluripotent stem cells (iPSCs) from schizophrenia patients and controls. Proteomic analyses were performed on the enriched mitochondrial and nuclear fractions of NSCs and neurons. Whole-cell proteomic analysis was also performed in neurons. Our results revealed alteration in proteins related to mitochondrial function, cell cycle control, among others. We also performed energy pathway analysis and reactive oxygen species (ROS) analysis of NSCs, which revealed alterations in mitochondrial oxygen consumption and a tendency of higher levels of intracellular ROS in schizophrenia-derived cells.







Similar content being viewed by others
Availability of data and materials
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE [84] partner repository with the dataset identifier PXD021976 and https://doi.org/10.6019/PXD021976.
References
Kahn RS, Sommer IE, Murray RM et al (2015) Schizophrenia. Nat Rev Dis Prim. https://doi.org/10.1038/nrdp.2015.67
Insel TR (2010) Rethinking schizophrenia. Nature 468:187–193. https://doi.org/10.1038/nature09552
Martins-de-Souza D, Harris LW, Guest PC, Bahn S (2011) The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal 15:2067–2079. https://doi.org/10.1089/ars.2010.3459
Nascimento JM, Martins-de-Souza D (2015) The proteome of schizophrenia. npj Schizophr 1:14003. https://doi.org/10.1038/npjschz.2014.3
Zuccoli GS, Saia-Cereda VM, Nascimento JM, Martins-de-Souza D (2017) The energy metabolism dysfunction in psychiatric disorders postmortem brains: Focus on proteomic evidence. Front Neurosci 11:1–14. https://doi.org/10.3389/fnins.2017.00493
Taylor SW, Fahy E, Ghosh SS (2003) Global Organellar Proteom 21:82–88
Pedrosa E, Sandler V, Shah A et al (2011) Development of patient-specific neurons in schizophrenia using induced pluripotent stem cells. J Neurogenet 25:88–103. https://doi.org/10.3109/01677063.2011.597908
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126:663–676. https://doi.org/10.1016/j.cell.2006.07.024
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872. https://doi.org/10.1016/j.cell.2007.11.019
Marchetto MC, Brennand KJ, Boyer LF, Gage FH (2011) Induced pluripotent stem cells (iPSCs) and neurological disease modeling: progress and promises. Hum Mol Genet 20:R109–R115. https://doi.org/10.1093/hmg/ddr336
Sebastian R, Song Y, Pak CH (2022) Probing the molecular and cellular pathological mechanisms of schizophrenia using human induced pluripotent stem cell models. Schizophr Res. https://doi.org/10.1016/j.schres.2022.06.028
Notaras M, Lodhi A, Fang H et al (2021) The proteomic architecture of schizophrenia iPSC-derived cerebral organoids reveals alterations in GWAS and neuronal development factors. Transl Psychiatry 11:1–16. https://doi.org/10.1038/s41398-021-01664-5
Notaras M, Lodhi A, Dündar F et al (2022) Schizophrenia is defined by cell-specific neuropathology and multiple neurodevelopmental mechanisms in patient-derived cerebral organoids. Mol Psychiatry 27:1416–1434. https://doi.org/10.1038/s41380-021-01316-6
Marchetto MCN, Winner B, Gage FH (2010) Pluripotent stem cells in neurodegenerative and neurodevelopmental diseases. Hum Mol Genet 19:R71–R76. https://doi.org/10.1093/hmg/ddq159
Brennand KJ, Simone A, Jou J et al (2011) Modelling schizophrenia using human induced pluripotent stem cells. Nature 473:221–225. https://doi.org/10.1038/nature09915
Brennand KJ, Marchetto MC, Benvenisty N et al (2015) Creating patient-specific neural cells for the in Vitro Study of Brain Disorders. Stem Cell Rep 5:933–945. https://doi.org/10.1016/j.stemcr.2015.10.011
Casas BS, Vitória G, Do Costa MN et al (2018) HiPSC-derived neural stem cells from patients with schizophrenia induce an impaired angiogenesis. Transl Psych. https://doi.org/10.1038/s41398-018-0095-9
Da Silveira B, De Paulsen R, Maciel M, Galina A et al (2012) Altered Oxygen Metabolism Associated to Neurogenesis of Induced Pluripotent Stem Cells Derived From a Schizophrenic Patient. Cell Transpl 21:1547–1559. https://doi.org/10.3727/096368911X600957
da Silveira B, Paulsen SC, Cardoso MP, Stelling, et al (2014) Valproate reverts zinc and potassium imbalance in schizophrenia-derived reprogrammed cells. Schizophr Res 154:30–35. https://doi.org/10.1016/j.schres.2014.02.007
Narula K, Datta A, Chakraborty N, Chakraborty S (2013) Comparative analyses of nuclear proteome: extending its function. Front Plant Sci 4:1–14. https://doi.org/10.3389/fpls.2013.00100
Khacho M, Slack RS (2018) Mitochondrial dynamics in the regulation of neurogenesis: From development to the adult brain. Dev Dyn 247:47–53. https://doi.org/10.1002/dvdy.24538
Hroudová J, Fišar Z (2011) Connectivity between mitochondrial functions and psychiatric disorders. Psychiatry Clin Neurosci 65:130–141
Rezin GT, Amboni G, Zugno AI et al (2009) Mitochondrial dysfunction and psychiatric disorders. Neurochem Res 34:1021–1029. https://doi.org/10.1007/s11064-008-9865-8
Trindade P, Nascimento JM, Casas BS et al (2023) Induced pluripotent stem cell-derived astrocytes from patients with schizophrenia exhibit an inflammatory phenotype that affects vascularization. Mol Psychiatry 28:871–882. https://doi.org/10.1038/s41380-022-01830-1
Sochacki J, Devalle S, Reis M et al (2016) Generation of iPS cell lines from schizophrenia patients using a non-integrative method. Stem Cell Res 17:97–101. https://doi.org/10.1016/j.scr.2016.05.017
Nascimento JM, Saia-Cereda VM, Zuccoli GS et al (2022) Proteomic signatures of schizophrenia-sourced iPSC-derived neural cells and brain organoids are similar to patients’ postmortem brains. Cell Biosci 12:1–16. https://doi.org/10.1186/s13578-022-00928-x
Clayton DA, Shadel GS (2014) Isolation of mitochondria from tissue culture cells. Cold Spring Harb Protoc 2014:1109–1111. https://doi.org/10.1101/pdb.prot080002
Shevchenko A, Tomas H, Havliš J et al (2007) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat Protoc 1:2856–2860. https://doi.org/10.1038/nprot.2006.468
Calvano SE, Xiao W, Richards DR et al (2005) A network-based analysis of systemic inflammation in humans. Nature 437:1032–1037. https://doi.org/10.1038/nature03985
Wu X, Al HM, Chen JY (2014) Pathway and network analysis in proteomics. J Theor Biol 362:44–52. https://doi.org/10.1016/j.jtbi.2014.05.031
Krämer A, Green J, Pollard J, Tugendreich S (2014) Causal analysis approaches in ingenuity pathway analysis. Bioinformatics 30:523–530. https://doi.org/10.1093/bioinformatics/btt703
Oparka M, Walczak J, Malinska D et al (2016) Quantifying ROS levels using CM-H2DCFDA and HyPer. Methods 109:3–11. https://doi.org/10.1016/j.ymeth.2016.06.008
Beckervordersandforth R, Ebert B, Schäffner I et al (2017) Role of mitochondrial metabolism in the control of early lineage progression and aging phenotypes in adult hippocampal neurogenesis. Neuron 93:560-573.e6. https://doi.org/10.1016/j.neuron.2016.12.017
Khacho M, Clark A, Svoboda DS et al (2017) Mitochondrial dysfunction underlies cognitive defects as a result of neural stem cell depletion and impaired neurogenesis. Hum Mol Genet 26:3327–3341. https://doi.org/10.1093/hmg/ddx217
Khacho M, Clark A, Svoboda DS et al (2016) Mitochondrial dynamics impacts stem cell identity and fate decisions by regulating a nuclear transcriptional program. Cell Stem Cell 19:232–247. https://doi.org/10.1016/j.stem.2016.04.015
Steib K, Schaffner I, Jagasia R et al (2014) Mitochondria modify exercise-induced development of stem cell-derived neurons in the adult brain. J Neurosci 34:6624–6633. https://doi.org/10.1523/JNEUROSCI.4972-13.2014
Candelario KM, Shuttleworth CW, Cunningham LA (2013) Neural stem/progenitor cells display a low requirement for oxidative metabolism independent of hypoxia inducible factor-1alpha expression. J Neurochem 125:420–429. https://doi.org/10.1111/jnc.12204
Studer L, Csete M, Lee SH et al (2000) Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci 20:7377–7383. https://doi.org/10.1523/jneurosci.20-19-07377.2000
Pistollato F, Chen HL, Schwartz PH et al (2007) Oxygen tension controls the expansion of human CNS precursors and the generation of astrocytes and oligodendrocytes. Mol Cell Neurosci 35:424–435. https://doi.org/10.1016/j.mcn.2007.04.003
Panchision DM (2009) The role of oxygen in regulating neural stem cells in development and disease. J Cell Physiol 220:562–568. https://doi.org/10.1002/jcp.21812
Bélanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14:724–738. https://doi.org/10.1016/j.cmet.2011.08.016
Kim D, Rhee I, Paik J (2014) Metabolic circuits in neural stem cells. Cell Mol Life Sci 71:4221–4241. https://doi.org/10.1007/s00018-014-1686-0
Kann O, Kovács R (2007) Mitochondria and neuronal activity. Am J Physiol Physiol 292:C641–C657. https://doi.org/10.1152/ajpcell.00222.2006
Rajasekaran A, Venkatasubramanian G, Berk M, Debnath M (2015) Mitochondrial dysfunction in schizophrenia: Pathways, mechanisms and implications. Neurosci Biobehav Rev 48:10–21. https://doi.org/10.1016/j.neubiorev.2014.11.005
Levy M, Faas GC, Saggau P et al (2003) Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem 278:17727–17734. https://doi.org/10.1074/jbc.M212878200
Nicholls DG, Budd SL (2000) Mitochondria and Neuronal Survival. Physiol Rev 80:315–360. https://doi.org/10.1152/physrev.2000.80.1.315
Ni P, Noh H, Park GH et al (2019) iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have compromised mitochondrial function. Mol Psychiatry. https://doi.org/10.1038/s41380-019-0423-3
Robicsek O, Karry R, Petit I et al (2013) Abnormal neuronal differentiation and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry 18:1067–1076. https://doi.org/10.1038/mp.2013.67
Cremisi F, Philpott A, Ohnuma SI (2003) Cell cycle and cell fate interactions in neural development. Curr Opin Neurobiol 13:26–33. https://doi.org/10.1016/S0959-4388(03)00005-9
Katsel P, Davis KL, Li C et al (2008) Abnormal indices of cell cycle activity in schizophrenia and their potential association with oligodendrocytes. Neuropsychopharmacology 33:2993–3009. https://doi.org/10.1038/npp.2008.19
Fan Y, Abrahamsen G, McGrath JJ, MacKay-Sim A (2012) Altered cell cycle dynamics in schizophrenia. Biol Psychiatry 71:129–135. https://doi.org/10.1016/j.biopsych.2011.10.004
Raza MU, Tufan T, Wang Y et al (2017) DNA damage in major psychiatric diseases. Neurotox Res 30:251–267. https://doi.org/10.1007/s12640-016-9621-9
Odemis S, Tuzun E, Gulec H et al (2016) Association between polymorphisms of DNA repair genes and risk of Schizophrenia. Genet Test Mol Biomark 20:11–17. https://doi.org/10.1089/gtmb.2015.0168
Saadat M, Pakyari N, Farrashbandi H (2008) Genetic polymorphism in the DNA repair gene XRCC1 and susceptibility to schizophrenia. Psychiatry Res 157:241–245. https://doi.org/10.1016/j.psychres.2007.07.014
Markkanen E, Meyer U, Dianov GL (2016) Dna damage and repair in schizophrenia and autism: Implications for cancer comorbidity and beyond. Int J Mol Sci. https://doi.org/10.3390/ijms17060856
Lee Y, McKinnon PJ (2007) Responding to DNA double strand breaks in the nervous system. Neuroscience 145:1365–1374. https://doi.org/10.1016/j.neuroscience.2006.07.026
Engelman JA, Luo J, Cantley LC (2006) The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet 7:606–619. https://doi.org/10.1038/nrg1879
Woodgett JR, Cohen P (1984) Multisite phosphorylation of glycogen synthase. Molecular basis for the substrate specificity of glycogen synthease kinase-3 and casein kinase-II (glycogen synthase kinase-5). Biochim Biophys Acta (BBA)/Protein Struct Mol 788:339–347. https://doi.org/10.1016/0167-4838(84)90047-5
Hur EM, Zhou FQ (2010) GSK3 signalling in neural development. Nat Rev Neurosci 11:539–551. https://doi.org/10.1038/nrn2870
Bijur GN, Jope RS (2003) Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J Neurochem 87:1427–1435. https://doi.org/10.1046/j.1471-4159.2003.02113.x
Ong SB, Hall AR, Dongworth RK et al (2015) Akt protects the heart against ischaemia-reperfusion injury by modulating mitochondrial morphology. Thromb Haemost 113:513–521. https://doi.org/10.1160/TH14-07-0592
Wang L, Zhou K, Fu Z et al (2017) Brain development and Akt Signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci 61:379–384. https://doi.org/10.1007/s12031-016-0872-y
Emamian ES (2012) AKT/GSK3 signaling pathway and schizophrenia. Front Mol Neurosci 5:1–12. https://doi.org/10.3389/fnmol.2012.00033
Polleux F, Snider W (2010) Initiating and growing an axon. Cold Spring Harb Perspect Biol 2:1–19. https://doi.org/10.1101/cshperspect.a001925
Da Silva JS, Dotti CG (2002) Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci 3:694–704. https://doi.org/10.1038/nrn918
Bellon A (2007) New genes associated with schizophrenia in neurite formation: A review of cell culture experiments. Mol Psychiatry 12:620–629. https://doi.org/10.1038/sj.mp.4001985
Kamiya A, Kubo KI, Tomoda T et al (2005) A schizophrenia-associated mutation of DISC1 perturbs cerebral cortex development. Nat Cell Biol 7:1067–1078. https://doi.org/10.1038/ncb1328
Ozeki Y, Tomoda T, Kleiderlein J et al (2003) Disrupted-in-Schizophrenia-1 (DISC-1): mutant truncation prevents binding to NudE-like (NUDEL) and inhibits neurite outgrowth. Proc Natl Acad Sci U S A 100:289–294. https://doi.org/10.1073/pnas.0136913100
Brennand KJ, Gage FH (2011) Concise review: the promise of human induced pluripotent stem cell-based studies of schizophrenia. Stem Cells 29:1915–1922. https://doi.org/10.1002/stem.762
Jones LB, Johnson N, Byne W (2002) Alterations in MAP2 immunocytochemistry in areas 9 and 32 of schizophrenic prefrontal cortex. Psychiatry Res Neuroimaging 114:137–148
Kalus P, Mu CATJ, Zuschratter W, Senitz D (2000) The dendritic architecture of prefrontal pyramidal neurons in schizophrenic patients. NeuroReport 11:3621–3625
Zheng X, Boyer L, Jin M et al (2016) Metabolic reprogramming during neuronal differentiation from aerobic glycolysis to neuronal oxidative phosphorylation. Elife 5:1–25. https://doi.org/10.7554/eLife.13374
Nugent AA, Kolpak AL, Engle EC (2012) Human disorders of axon guidance. Curr Opin Neurobiol 22:837–843. https://doi.org/10.1016/j.conb.2012.02.006
Korecka JA, Levy S, Isacson O (2016) In vivo modeling of neuronal function, axonal impairment and connectivity in neurodegenerative and neuropsychiatric disorders using induced pluripotent stem cells. Mol Cell Neurosci 73:3–12. https://doi.org/10.1016/j.mcn.2015.12.004
Gilman SR, Chang J, Xu B et al (2012) Diverse types of genetic variation converge on functional gene networks involved in schizophrenia. Nat Neurosci 15:1723–1728. https://doi.org/10.1038/nn.3261
Lewis D, a, Lieberman J a, (2000) Catching up on schizophrenia: natural history and neurobiology. Neuron 28:325–334. https://doi.org/10.1016/S0896-6273(00)00111-2
Smith GM, Gallo G (2018) The role of mitochondria in axon development and regeneration. Dev Neurobiol 78:221–237. https://doi.org/10.1002/dneu.22546
Do KQ, Cabungcal JH, Frank A et al (2009) Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol 19:220–230. https://doi.org/10.1016/j.conb.2009.05.001
Behrens MM, Ali SS, Dugan LL (2008) Interleukin-6 mediates the increase in NADPH-oxidase in the ketamine model of Schizophrenia. J Neurosci 28:13957–13966. https://doi.org/10.1523/JNEUROSCI.4457-08.2008
Pedrini M, Massuda R, Fries GR et al (2012) Similarities in serum oxidative stress markers and inflammatory cytokines in patients with overt schizophrenia at early and late stages of chronicity. J Psychiatr Res 46:819–824. https://doi.org/10.1016/j.jpsychires.2012.03.019
Wells PG, Mccallum GP, Chen CS et al (2009) Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci 108:4–18. https://doi.org/10.1093/toxsci/kfn263
Liu G, Dwyer T (2014) Microtubule dynamics in axon guidance. Neurosci Bull 30:569–583. https://doi.org/10.1007/s12264-014-1444-6
Pacheco A, Gallo G (2016) Actin filament-microtubule interactions in axon initiation and branching. Brain Res Bull 126:300–310. https://doi.org/10.1016/j.brainresbull.2016.07.013
Perez-Riverol Y, Csordas A, Bai J et al (2019) The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res 47:D442–D450. https://doi.org/10.1093/nar/gky1106
Acknowledgements
We thank Gabriela Lopes Vitória for her excellent support related to stem cell generation and culture and Ludmila S. S. Bastos for cell bank expansion. We also thank Mariana Fioramonte for her support with the mass spectrometry analysis. We thank the São Paulo Research Foundation—FAPESP (grant numbers 2016/04912-2, 2018/14666-4, 2014/21035-0, 2015/15626-8, 2017/25588-1 and 2019/00098-7); the Brazilian National Council for Scientific and Technological Development—CNPq and the Foundation for Research Support in the State of Rio de Janeiro (FAPERJ) for funding this work.
Funding
Conselho Nacional de Desenvolvimento Científico e Tecnológico, 2018/01410-1, Daniel Martins de-Souza, Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro.
Author information
Authors and Affiliations
Contributions
GSZ, JMN, SKR and DMS contributed to the conceptualization and design of the study. GSZ performed the experiments and analyzed the data. PMMV contributed to the design of the metabolic analysis. GSZ wrote the first draft of the manuscript. GSZ, JMN and DMS reviewed the final manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zuccoli, G.S., Nascimento, J.M., Moraes-Vieira, P.M. et al. Mitochondrial, cell cycle control and neuritogenesis alterations in an iPSC-based neurodevelopmental model for schizophrenia. Eur Arch Psychiatry Clin Neurosci 273, 1649–1664 (2023). https://doi.org/10.1007/s00406-023-01605-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-023-01605-x