Abstract
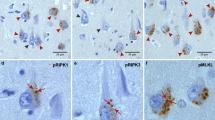
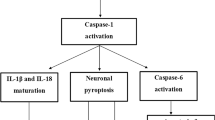
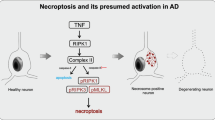
The major neuropathological hallmarks of Alzheimer’s disease (AD) are amyloid β (Aβ) plaques and neurofibrillary tangles (NFT), accompanied by neuroinflammation and neuronal loss. Increasing evidence is emerging for the activation of the canonical NOD-, LRR- and pyrin domain-containing 3 (NLRP3) inflammasome in AD. However, the mechanisms leading to neuronal loss in AD and the involvement of glial cells in these processes are still not clear. The aim of this study was to investigate the contribution of pyroptosis, a pro-inflammatory mechanism of cell death downstream of the inflammasome, to neurodegeneration in AD. Immunohistochemistry and biochemical analysis of protein levels were performed on human post-mortem brain tissue. We investigated the presence of cleaved gasdermin D (GSDMD), the pyroptosis effector protein, as well as the NLRP3 inflammasome-forming proteins, in the medial temporal lobe of 23 symptomatic AD, 25 pathologically defined preclinical AD (p-preAD) and 21 non-demented control cases. Cleaved GSDMD was detected in microglia, but also in astrocytes and in few pyramidal neurons in the first sector of the cornu ammonis (CA1) of the hippocampus and the temporal cortex of Brodmann area 36. Only microglia expressed all NLRP3 inflammasome-forming proteins (i.e., ASC, NLRP3, caspase-1). Cleaved GSDMD-positive astrocytes and neurons exhibited caspase-8 and non-canonical inflammasome protein caspase-4, respectively, potentially indicating alternative pathways for GSDMD cleavage. Brains of AD patients exhibited increased numbers of cleaved GSDMD-positive cells. Cleaved GSDMD-positive microglia and astrocytes were found in close proximity to Aβ plaques, while cleaved GSDMD-positive neurons were devoid of NFTs. In CA1, NLRP3-positive microglia and cleaved GSDMD-positive neurons were associated with local neuronal loss, indicating a possible contribution of NLRP3 inflammasome and pyroptosis activation to AD-related neurodegeneration. Taken together, our results suggest cell type-specific activation of pyroptosis in AD and extend the current knowledge about the contribution of neuroinflammation to the neurodegenerative process in AD via a direct link to neuron death by pyroptosis.








Similar content being viewed by others
Availability of data and material
All relevant data generated and analyzed in this study are available in this manuscript, online supplementary information or upon reasonable request.
References
Arranz AM, De Strooper B (2019) The role of astroglia in Alzheimer’s disease: pathophysiology and clinical implications. Lancet Neurol 18:406–414. https://doi.org/10.1016/S1474-4422(18)30490-3
Auer RN, Wieloch T, Olsson Y, Siesjö BK (1984) The distribution of hypoglycemic brain damage. Acta Neuropathol 64:177–191. https://doi.org/10.1007/BF00688108
Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Tredici K (2006) Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol 112:389–404. https://doi.org/10.1007/s00401-006-0127-z
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259. https://doi.org/10.1007/bf00308809
Cacace R, Sleegers K, Van BC (2016) Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimer’s Dement 12:733–748. https://doi.org/10.1016/j.jalz.2016.01.012
Caccamo A, Branca C, Piras IS, Ferreira E, Huentelman MJ, Liang WS et al (2017) Necroptosis activation in Alzheimer’s disease. Nat Neurosci 20:1236–1246. https://doi.org/10.1038/nn.4608
Cavalcante GC, Schaan AP, Cabral GF, Santana-Da-Silva MN, Pinto P, Vidal AF et al (2019) A cell’s fate: an overview of the molecular biology and genetics of apoptosis. Int J Mol Sci 20:4133. https://doi.org/10.3390/ijms20174133
Coppé JP, Desprez PY, Krtolica A, Campisi J (2010) The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol Mech Dis 5:99–118. https://doi.org/10.1146/annurev-pathol-121808-102144
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. https://doi.org/10.1007/s00401-014-1349-0
de Vasconcelos NM, Van Opdenbosch N, Van Gorp H, Parthoens E, Lamkanfi M (2019) Single-cell analysis of pyroptosis dynamics reveals conserved GSDMD-mediated subcellular events that precede plasma membrane rupture. Cell Death Differ 26:146–161. https://doi.org/10.1038/s41418-018-0106-7
Felleman DJ, Van Essen DC (1991) Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex 1:1–47. https://doi.org/10.1093/cercor/1.1.1-a
Fricker M, Tolkovsky AM, Borutaite V, Coleman M, Brown GC (2018) Neuronal cell death. Physiol Rev 98:813–880. https://doi.org/10.1152/physrev.00011.2017
Friker LL, Scheiblich H, Hochheiser IV, Brinkschulte R, Riedel D, Latz E et al (2020) β-amyloid clustering around ASC fibrils boosts its toxicity in microglia. Cell Rep 30:3743–3754. https://doi.org/10.1016/j.celrep.2020.02.025
Fritsch M, Günther SD, Schwarzer R, Albert MC, Schorn F, Werthenbach JP et al (2019) Caspase-8 is the molecular switch for apoptosis, necroptosis and pyroptosis. Nature 575:683–687. https://doi.org/10.1038/s41586-019-1770-6
García-Cabezas M, John YJ, Barbas H, Zikopoulos B (2016) Distinction of neurons, glia and endothelial cells in the cerebral cortex: an algorithm based on cytological features. Front Neuroanat 10:107. https://doi.org/10.3389/fnana.2016.00107
Gomes LA, Hipp SA, Rijal Upadhaya A, Balakrishnan K, Ospitalieri S, Koper MJ et al (2019) Aβ-induced acceleration of Alzheimer-related τ-pathology spreading and its association with prion protein. Acta Neuropathol 138:913–941. https://doi.org/10.1007/s00401-019-02053-5
Götz J, Chen F, Van Dorpe J, Nitsch RM (2001) Formation of neurofibrillary tangles in P301L tau transgenic mice induced by Aβ42 fibrils. Science 293:1491–1495. https://doi.org/10.1126/science.1062097
Greenwood EK, Brown DR (2021) Senescent microglia: the key to the ageing brain? Int J Mol Sci 22:4402. https://doi.org/10.3390/ijms22094402
Griffin WS, Sheng JG, Roberts GW, Mrak RE (1995) Interleukin-1 expression in different plaque types in Alzheimerʼs disease. J Neuropathol Exp Neurol 54:276–281. https://doi.org/10.1097/00005072-199503000-00014
Halle A, Hornung V, Petzold GC, Stewart CR, Monks BG, Reinheckel T et al (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat Immunol 9:857–865. https://doi.org/10.1038/ni.1636
Hammond TR, Dufort C, Dissing-Olesen L, Giera S, Young A, Wysoker A et al (2019) Single-cell RNA sequencing of microglia throughout the mouse lifespan and in the injured brain reveals complex cell-state changes. Immunity 50:253-271.e6. https://doi.org/10.1016/j.immuni.2018.11.004
Han C, Yang Y, Guan Q, Zhang X, Shen H, Sheng Y et al (2020) New mechanism of nerve injury in Alzheimer’s disease: β-amyloid-induced neuronal pyroptosis. J Cell Mol Med 24:8078–8090. https://doi.org/10.1111/jcmm.15439
He W, Wan H, Hu L, Chen P, Wang X, Huang Z et al (2015) Gasdermin D is an executor of pyroptosis and required for interleukin-1 β secretion. Cell Res 25:1285–1298. https://doi.org/10.1038/cr.2015.139
Hecht M, Krämer LM, von Arnim CAF, Otto M, Thal DR (2018) Capillary cerebral amyloid angiopathy in Alzheimer’s disease: association with allocortical/hippocampal microinfarcts and cognitive decline. Acta Neuropathol 135:681–694. https://doi.org/10.1007/s00401-018-1834-y
Heneka MT, Kummer MP, Stutz A, Delekate A, Schwartz S, Vieira-Saecker A et al (2013) NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 493:674–678. https://doi.org/10.1038/nature11729
Heneka MT, McManus RM, Latz E (2018) Inflammasome signalling in brain function and neurodegenerative disease. Nat Rev Neurosci 19:610–621. https://doi.org/10.1038/s41583-018-0055-7
Hitomi J, Katayama T, Eguchi Y, Kudo T, Taniguchi M, Koyama Y et al (2004) Involvement of caspase-4 in endoplasmic reticulum stress-induced apoptosis and Aβ-induced cell death. J Cell Biol 165:347–356. https://doi.org/10.1083/jcb.200310015
Hong Y, Liu Y, Yu D, Wang M, Hou Y (2019) The neuroprotection of progesterone against Aβ-induced NLRP3-Caspase-1 inflammasome activation via enhancing autophagy in astrocytes. Int Immunopharmacol 74:105669. https://doi.org/10.1016/j.intimp.2019.05.054
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J et al (2020) FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol 21:736–745. https://doi.org/10.1038/s41590-020-0669-6
Hu Y, Fryatt GL, Ghorbani M, Obst J, Menassa DA, Martin-Estebane M et al (2021) Replicative senescence dictates the emergence of disease-associated microglia and contributes to Aβ pathology. Cell Rep 35:109228. https://doi.org/10.1016/j.celrep.2021.109228
Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140:566–572. https://doi.org/10.1192/bjp.140.6.566
Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC et al (2012) National institute on aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s Dement 8:1–13. https://doi.org/10.1016/j.jalz.2011.10.007
Hyman BT, Van Hoesen GW, Damasio AR, Barnes CL (1984) Alzheimer’s disease: cell-specific pathology isolates the hippocampal formation. Science 225:1168–1170. https://doi.org/10.1126/science.6474172
Ising C, Venegas C, Zhang S, Scheiblich H, Schmidt SV, Vieira-saecker A et al (2019) NLRP3 inflammasome activation drives tau pathology. Nature 575:669–673. https://doi.org/10.1038/s41586-019-1769-z
Kajiwara Y, McKenzie A, Dorr N, Gama Sosa MA, Elder G, Schmeidler J et al (2016) The human-specific CASP4 gene product contributes to Alzheimer-related synaptic and behavioural deficits. Hum Mol Genet 25:4315–4327. https://doi.org/10.1093/hmg/ddw265
Kayagaki N, Warming S, Lamkanfi M, Vande WL, Louie S, Dong J et al (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. https://doi.org/10.1038/nature10558
Keren-Shaul H, Spinrad A, Weiner A, Matcovitch-Natan O, Dvir-Szternfeld R, Ulland TK et al (2017) A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169:1276-1290.e17. https://doi.org/10.1016/j.cell.2017.05.018
Kinney JW, Bemiller SM, Murtishaw AS, Leisgang AM, Salazar AM, Lamb BT (2018) Inflammation as a central mechanism in Alzheimer’s disease. Alzheimer’s Dement Transl Res Clin Interv 4:575–590. https://doi.org/10.1016/j.trci.2018.06.014
Köhler C, Dinekov M, Götz J (2014) Granulovacuolar degeneration and unfolded protein response in mouse models of tauopathy and Aβ amyloidosis. Neurobiol Dis 71:169–179. https://doi.org/10.1016/j.nbd.2014.07.006
Koper MJ, Van Schoor E, Ospitalieri S, Vandenberghe R, Vandenbulcke M, von Arnim CAF et al (2020) Necrosome complex detected in granulovacuolar degeneration is associated with neuronal loss in Alzheimer’s disease. Acta Neuropathol 139:463–484. https://doi.org/10.1007/s00401-019-02103-y
Lamkanfi M, Dixit VM (2014) Mechanisms and functions of inflammasomes. Cell 157:1013–1022. https://doi.org/10.1016/j.cell.2014.04.007
Latz E, Xiao TS, Stutz A (2013) Activation and regulation of the inflammasomes. Nat Rev Immunol 13:397–411. https://doi.org/10.1038/nri3452
Lewis J, Dickson DW, Lin WL, Chisholm L, Corral A, Jones G et al (2001) Enhanced neurofibrillary degeneration in transgenic mice expressing mutant tau and APP. Science 293:1487–1491. https://doi.org/10.1126/science.1058189
Mancuso R, Fattorelli N, Martinez-Muriana A, Davis E, Wolfs L, Van Den Daele J et al (2022) A multi-pronged human microglia response to Alzheimer’s disease Aβ pathology. bioRxiv. https://doi.org/10.1101/2022.07.07.499139
Masters CL, Bateman R, Blennow K, Rowe CC, Sperling RA, Cummings JL (2015) Alzheimer’s disease. Nat Rev Dis Prim 1:15056. https://doi.org/10.1038/nrdp.2015.56
McKenzie BA, Mamik MK, Saito LB, Boghozian R, Monaco MC, Major EO et al (2018) Caspase-1 inhibition prevents glial inflammasome activation and pyroptosis in models of multiple sclerosis. Proc Natl Acad Sci U S A 115:E6065–E6074. https://doi.org/10.1073/pnas.1722041115
Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM et al (1991) The consortium to establish a registry for Alzheimer’s disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486. https://doi.org/10.1212/wnl.41.4.479
Nishizaki T (2019) Fe3+ Facilitates endocytic internalization of extracellular Aβ1–42 and enhances Aβ1–42 -induced caspase-3/caspase-4 activation and neuronal cell death. Mol Neurobiol 56:4812–4819. https://doi.org/10.1007/s12035-018-1408-y
Ransohoff RM (2016) How neuroinflammation contributes to neurodegeneration. Science 353:777–783. https://doi.org/10.1126/science.aag2590
Rathkey JK, Zhao J, Liu Z, Chen Y, Yang J, Hannah C et al (2018) Chemical disruption of the pyroptotic pore-forming protein gasdermin D inhibits inflammatory cell death and sepsis. Sci Immunol 3:eaat2738. https://doi.org/10.1126/sciimmunol.aat2738.Chemical
Ratner B (2009) The correlation coefficient: its values range between 1/1, or do they. J Target Meas Anal Mark 17:139–142. https://doi.org/10.1057/jt.2009.5
Rijal Upadhaya A, Kosterin I, Kumar S, von Arnim CAF, Yamaguchi H, Fändrich M et al (2014) Biochemical stages of amyloid-β peptide aggregation and accumulation in the human brain and their association with symptomatic and pathologically preclinical Alzheimer’s disease. Brain 137:887–903. https://doi.org/10.1093/brain/awt362
Rohn TT, Head E, Nesse WH, Cotman CW, Cribbs DH (2001) Activation of caspase-8 in the Alzheimer’s disease brain. Neurobiol Dis 8:1006–1016. https://doi.org/10.1006/nbdi.2001.0449
Rolyan H, Scheffold A, Heinrich A, Begus-Nahrmann Y, Langkopf BH, Hölter SM et al (2011) Telomere shortening reduces Alzheimer’s disease amyloid pathology in mice. Brain 134:2044–2056. https://doi.org/10.1093/brain/awr133
Rühl S, Shkarina K, Demarco B, Heilig R, Santos JC, Broz P (2018) ESCRT-dependent membrane repair negatively regulates pyroptosis downstream of GSDMD activation. Science 362:956–960. https://doi.org/10.1126/science.aar7607
Sarhan J, Liu BC, Muendlein HI, Li P, Nilson R, Tang AY et al (2018) Caspase-8 induces cleavage of gasdermin D to elicit pyroptosis during Yersinia infection. Proc Natl Acad Sci U S A 115:E10888–E10897. https://doi.org/10.1073/pnas.1809548115
Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H et al (2016) GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J 35:1766–1778. https://doi.org/10.15252/embj.201694696
Schaeverbeke JM, Gabel S, Meersmans K, Luckett ES, De Meyer S, Adamczuk K et al (2021) Baseline cognition is the best predictor of 4-year cognitive change in cognitively intact older adults. Alzheimer’s Res Ther 13:75. https://doi.org/10.1186/s13195-021-00798-4
Serrano-Pozo A, Mielke ML, Gómez-Isla T, Betensky RA, Growdon JH, Frosch MP et al (2011) Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. AJPA 179:1373–1384. https://doi.org/10.1016/j.ajpath.2011.05.047
Shen H, Han C, Yang Y, Guo L, Sheng Y, Wang J et al (2021) Pyroptosis executive protein GSDMD as a biomarker for diagnosis and identification of Alzheimer’s disease. Brain Behav 11:e02063. https://doi.org/10.1002/brb3.2063
Shi J, Gao W, Shao F (2017) Pyroptosis: gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci 42:245–254. https://doi.org/10.1016/j.tibs.2016.10.004
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H et al (2015) Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526:660–665. https://doi.org/10.1038/nature15514
Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P et al (2014) Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192. https://doi.org/10.1038/nature13683
Sierksma A, Lu A, Mancuso R, Fattorelli N, Thrupp N, Salta E et al (2020) Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Mol Med 12:e10606. https://doi.org/10.15252/emmm.201910606
Song L, Pei L, Yao S, Wu Y, Shang Y (2017) NLRP3 inflammasome in neurological diseases, from functions to therapies. Front Cell Neurosci 11:63. https://doi.org/10.3389/fncel.2017.00063
Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM et al (2011) Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement 7:280–292. https://doi.org/10.1016/j.jalz.2011.03.003
Stadelmann C, Deckwerth TL, Srinivasan A, Bancher C, Brück W, Jellinger K et al (1999) Activation of caspase-3 in single neurons and autophagic granules of granulovacuolar degeneration in Alzheimer’s disease: Evidence for apoptotic cell death. Am J Pathol 155:1459–1466. https://doi.org/10.1016/S0002-9440(10)65460-0
Stancu IC, Cremers N, Vanrusselt H, Couturier J, Vanoosthuyse A, Kessels S et al (2019) Aggregated Tau activates NLRP3–ASC inflammasome exacerbating exogenously seeded and non-exogenously seeded Tau pathology in vivo. Acta Neuropathol 137:599–617. https://doi.org/10.1007/s00401-018-01957-y
Stence N, Waite M, Dailey ME (2001) Dynamics of microglial activation: a confocal time-lapse analysis in hippocampal slices. Glia 33:256–266. https://doi.org/10.1002/1098-1136(200103)33:3%3c256::AID-GLIA1024%3e3.0.CO;2-J
Su JH, Zhao M, Anderson AJ, Srinivasan A, Cotman CW (2001) Activated caspase-3 expression in Alzheimer’s and aged control brain: Correlation with Alzheimer pathology. Brain Res 898:350–357. https://doi.org/10.1016/S0006-8993(01)02018-2
Tang Y, Le W (2016) Differential roles of M1 and M2 microglia in neurodegenerative diseases. Mol Neurobiol 53:1181–1194. https://doi.org/10.1007/s12035-014-9070-5
Thal DR, Del Tredici K, Ludolph AC, Hoozemans JJM, Rozemuller AJ, Braak H et al (2011) Stages of granulovacuolar degeneration: their relation to Alzheimer’s disease and chronic stress response. Acta Neuropathol 122:577–589. https://doi.org/10.1007/s00401-011-0871-6
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800. https://doi.org/10.1212/WNL.58.12.1791
Thal DR, Rüb U, Schultz C, Sassin I, Ghebremedhin E, Del Tredici K et al (2000) Sequence of Aβ-protein deposition in the human medial temporal lobe. J Neuropathol Exp Neurol 59:733–748. https://doi.org/10.1093/jnen/59.8.733
Uedufy (2022) How To Run Mediation Analysis in SPSS [2 Methods]. https://uedufy.com/how-to-run-mediation-analysis-in-spss/. Accessed 18 Jul 2022
Van Schoor E, Ospitalieri S, Moonen S, Tomé SO, Ronisz A, Ok O et al (2022) Increased pyroptosis activation in white matter microglia is associated with neuronal loss in ALS motor cortex. Acta Neuropathol 144:393–411. https://doi.org/10.1007/s00401-022-02466-9
Venegas C, Kumar S, Franklin BS, Dierkes T, Brinkschulte R, Tejera D et al (2017) Microglia-derived ASC specks cross seed amyloid-β in Alzheimer’s disease. Nature 552:355–361. https://doi.org/10.1038/nature25158
West MJ, Coleman PD, Flood DG, Troncoso JC (1994) Differences in the pattern of hippocampal neuronal loss in normal ageing and Alzheimer ’ s disease. Lancet 344:769–772. https://doi.org/10.1016/s0140-6736(94)92338-8
Wiersma VI, Ziel Van AM, Vazquez S, Anna S, Ernesto N, Correa B et al (2019) Granulovacuolar degeneration bodies are neuron-selective lysosomal structures induced by intracellular tau pathology. Acta Neuropathol 138:943–970. https://doi.org/10.1007/s00401-019-02046-4
World Health Organization (2017) Global action plan on the public health response to dementia 2017–2025. World Health Organization, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/259615
Zahid A, Ismail H, Jin T (2021) Molecular and structural aspects of gasdermin family pores and insights into gasdermin-elicited programmed cell death. Biochem Soc Trans 49:2697–2710. https://doi.org/10.1042/BST20210672
Zarow C, Vinters HV, Ellis WG, Weiner MW, Mungas D, White L et al (2005) Correlates of hippocampal neuron number in Alzheimer’s disease and ischemic vascular dementia. Ann Neurol 57:896–903. https://doi.org/10.1002/ana.20503
Zendedel A, Johann S, Mehrabi S, Joghataei MT, Hassanzadeh G, Kipp M et al (2016) Activation and Regulation of NLRP3 Inflammasome by Intrathecal Application of SDF-1a in a Spinal Cord Injury Model. Mol Neurobiol 53:3063–3075. https://doi.org/10.1007/s12035-015-9203-5
Zhang X, Wang R, Hu D, Sun X, Fujioka H, Lundberg K et al (2020) Oligodendroglial glycolytic stress triggers inflammasome activation and neuropathology in Alzheimer’s disease. Sci Adv 6:eabb8680. https://doi.org/10.1126/sciadv.abb8680
Acknowledgements
This study was supported by funding from FWO-Vlaanderen (G0F8516N and G065721N, DRT). SM received a Doctoral mandate from KU Leuven internal funds (DB/20/007/BM). An SB PhD Fellowship from FWO-Vlaanderen was given to EVS (1S46219N). JMS is a junior postdoctoral fellow of the Fonds Wetenschappelijk Onderzoek (FWO/Belgium) (12Y1620N) and received funding from Stichting Alzheimer association (#SAO-FRA 2021/00022). We gratefully acknowledge the support of Ms. Simona Ospitalieri and Mrs. Alicja Ronisz, and the VIB Bio Imaging Core for their support & assistance in this study.
Author information
Authors and Affiliations
Contributions
SM: study design, neuropathology, immunohistochemistry, microscopic assessments, statistical analysis, manuscript drafting and preparation. MK: study design, immunohistochemistry, microscopic assessments, critical review of the manuscript. EVS: immunohistochemistry, critical review of the manuscript. JMS: statistical analysis, critical review of the manuscript. RV, CAFvA: clinical neurology, recruitment, critical review of the manuscript. TT: neuropathology and critical review of the manuscript. BDS: study design, critical review of the manuscript. DRT: study design and coordination, neuropathology, microscopic assessments, supplementary statistical analysis, and manuscript preparation. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
DRT is a member of the Acta Neuropathologica Editorial Board. He was not involved in the assessment or decision-making process for this manuscript. DRT received speaker honorary or travel reimbursement from UCB (Brussels, Belgium) and Biogen (USA), and collaborated with Novartis Pharma AG (Basel, Switzerland), Probiodrug (Halle (Saale), Germany), GE Healthcare (Amersham, UK), and Janssen Pharmaceutical Companies (Beerse, Belgium). RV’s institution has a clinical trial agreement (RV as PI) with Alector, Biogen, J&J, NovoNordisk, Prevail, Roche and UCB. RV’s institution has a consultancy agreement for participation in Data Safety Monitoring Board (RV as provider) with AC Immune and Novartis. RV’s institution has a material transfer agreement (RV as PI) with ADx Neurosciences. CAFvA received honoraria from serving on the scientific advisory board of Biogen, Roche, Novo Nordisk, and Dr. Willmar Schwabe GmbH &Co. KG and has received funding for travel and speaker honoraria from Biogen, Roche diagnostics AG, Medical Tribune Verlagsgesellschaft mbH, and Dr. Willmar Schwabe GmbH &Co. KG and has received research support from Roche diagnostics AG. Bart De Strooper is occasionally consulting for different companies. He is founding scientist of Augustin TX and of Muna TX. He is also shareholder of Muna TX. Funders were not involved in the design of this study, in the collection, analysis or interpretation of data, in the decision to publish the results, or in the preparation of the manuscript.
Ethical approval
Human post-mortem brain tissue was collected in accordance with the applicable laws in Belgium (UZ Leuven) and Germany (Ulm). The recruitment protocols for the collection of human brains were approved by the ethical committees of the UZ Leuven (Belgium; S-59292, S-52791) and the University of Ulm (Germany; 54/08). This study was approved by the UZ Leuven ethical committee (Leuven, Belgium; S-64378).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Moonen, S., Koper, M.J., Van Schoor, E. et al. Pyroptosis in Alzheimer’s disease: cell type-specific activation in microglia, astrocytes and neurons. Acta Neuropathol 145, 175–195 (2023). https://doi.org/10.1007/s00401-022-02528-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-022-02528-y