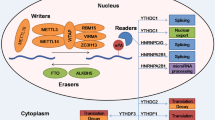
Abstract
N6-methyladenosine (m6A) RNA modification has recently emerged as an essential regulator of normal and malignant hematopoiesis. As a reversible epigenetic modification found in messenger RNAs and non-coding RNAs, m6A affects the fate of the modified RNA molecules. It is essential in most vital bioprocesses, contributing to cancer development. Here, we review the up-to-date knowledge of the pathological functions and underlying molecular mechanism of m6A modifications in normal hematopoiesis, leukemia pathogenesis, and drug response/resistance. At last, we discuss the critical role of m6A in immune response, the therapeutic potential of targeting m6A regulators, and the possible combination therapy for AML.




Similar content being viewed by others
References
De Kouchkovsky I, Abdul-Hay M (2016) Acute myeloid leukemia: a comprehensive review and 2016 update. Blood Cancer J. 6(7):e441. https://doi.org/10.1038/bcj.2016.50
Global Burden of Disease Cancer C, Fitzmaurice C, Abate D et al (2019) Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol 5(12):1749–1768. https://doi.org/10.1001/jamaoncol.2019.2996
Wouters BJ, Delwel R (2016) Epigenetics and approaches to targeted epigenetic therapy in acute myeloid leukemia. Blood 127(1):42–52. https://doi.org/10.1182/blood-2015-07-604512
Pan Y, Ma P, Liu Y et al (2018) Multiple functions of m(6)A RNA methylation in cancer. J Hematol Oncol 11(1):48. https://doi.org/10.1186/s13045-018-0590-8
Liu L, Li H, Hu D et al (2022) Insights into N6-methyladenosine and programmed cell death in cancer. Mol Cancer 21(1):32. https://doi.org/10.1186/s12943-022-01508-w
Wang Q, Chen C, Ding Q et al (2020) METTL3-mediated m(6)A modification of HDGF mRNA promotes gastric cancer progression and has prognostic significance. Gut 69(7):1193–1205. https://doi.org/10.1136/gutjnl-2019-319639
Zhang C, Samanta D, Lu H et al (2016) Hypoxia induces the breast cancer stem cell phenotype by HIF-dependent and ALKBH5-mediated m(6)A-demethylation of NANOG mRNA. Proc Natl Acad Sci U S A 113(14):E2047–E2056. https://doi.org/10.1073/pnas.1602883113
Qian X, Yang J, Qiu Q et al (2021) LCAT3, a novel m6A-regulated long non-coding RNA, plays an oncogenic role in lung cancer via binding with FUBP1 to activate c-MYC. J Hematol Oncol 14(1):112. https://doi.org/10.1186/s13045-021-01123-0
Chen Y, Peng C, Chen J et al (2019) WTAP facilitates progression of hepatocellular carcinoma via m6A-HuR-dependent epigenetic silencing of ETS1. Mol Cancer 18(1):127. https://doi.org/10.1186/s12943-019-1053-8
Gu C, Wang Z, Zhou N et al (2019) Mettl14 inhibits bladder TIC self-renewal and bladder tumorigenesis through N(6)-methyladenosine of Notch1. Mol Cancer 18(1):168. https://doi.org/10.1186/s12943-019-1084-1
Dominissini D, Moshitch-Moshkovitz S, Schwartz S et al (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A-seq. Nature 485(7397):201–206. https://doi.org/10.1038/nature11112
Meyer KD, Saletore Y, Zumbo P et al (2012) Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell 149(7):1635–1646. https://doi.org/10.1016/j.cell.2012.05.003
Fedeles BI, Singh V, Delaney JC et al (2015) The AlkB family of Fe(II)/alpha-ketoglutarate-dependent dioxygenases: repairing nucleic acid alkylation damage and beyond. J Biol Chem 290(34):20734–20742. https://doi.org/10.1074/jbc.R115.656462
Huang H, Weng H, Sun W et al (2018) Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat Cell Biol 20(3):285–295. https://doi.org/10.1038/s41556-018-0045-z
Xiao W, Adhikari S, Dahal U et al (2016) Nuclear m(6)A reader YTHDC1 regulates mRNA splicing. Mol Cell 61(4):507–519. https://doi.org/10.1016/j.molcel.2016.01.012
Liu N, Dai Q, Zheng G et al (2015) N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature 518(7540):560–564. https://doi.org/10.1038/nature14234
Edens BM, Vissers C, Su J et al (2019) FMRP modulates neural differentiation through m(6)A-dependent mRNA nuclear export. Cell Rep 28(4):845–854 e5. https://doi.org/10.1016/j.celrep.2019.06.072
Zaccara S, Ries RJ, Jaffrey SR (2019) Reading, writing and erasing mRNA methylation. Nat Rev Mol Cell Biol 20(10):608–624. https://doi.org/10.1038/s41580-019-0168-5
Narayan P, Rottman FM (1988) An in vitro system for accurate methylation of internal adenosine residues in messenger RNA. Science 242(4882):1159–1162. https://doi.org/10.1126/science.3187541
Doxtader KA, Wang P, Scarborough AM et al (2018) Structural basis for regulation of METTL16, an S-adenosylmethionine homeostasis factor. Mol Cell 71(6):1001–1011 e4. https://doi.org/10.1016/j.molcel.2018.07.025
Pendleton KE, Chen B, Liu K et al (2017) The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169(5):824–835 e14. https://doi.org/10.1016/j.cell.2017.05.003
Warda AS, Kretschmer J, Hackert P et al (2017) Human METTL16 is a N(6)-methyladenosine (m(6)A) methyltransferase that targets pre-mRNAs and various non-coding RNAs. EMBO Rep 18(11):2004–2014. https://doi.org/10.15252/embr.201744940
Ping XL, Sun BF, Wang L et al (2014) Mammalian WTAP is a regulatory subunit of the RNA N6-methyladenosine methyltransferase. Cell Res 24(2):177–189. https://doi.org/10.1038/cr.2014.3
Miao R, Dai CC, Mei L et al (2020) KIAA1429 regulates cell proliferation by targeting c-Jun messenger RNA directly in gastric cancer. J Cell Physiol 235(10):7420–7432. https://doi.org/10.1002/jcp.29645
Hu Y, Ouyang Z, Sui X et al (2020) Oocyte competence is maintained by m(6)A methyltransferase KIAA1429-mediated RNA metabolism during mouse follicular development. Cell Death Differ 27(8):2468–2483. https://doi.org/10.1038/s41418-020-0516-1
Patil DP, Chen CK, Pickering BF et al (2016) m(6)A RNA methylation promotes XIST-mediated transcriptional repression. Nature 537(7620):369–373. https://doi.org/10.1038/nature19342
Huang Y, Yan J, Li Q et al (2015) Meclofenamic acid selectively inhibits FTO demethylation of m6A over ALKBH5. Nucleic Acids Res 43(1):373–384. https://doi.org/10.1093/nar/gku1276
Wei J, Liu F, Lu Z et al (2018) Differential m(6)A, m(6)Am, and m(1)A Demethylation mediated by FTO in the cell nucleus and cytoplasm. Mol Cell 71(6):973–985 e5. https://doi.org/10.1016/j.molcel.2018.08.011
Bartosovic M, Molares HC, Gregorova P et al (2017) N6-methyladenosine demethylase FTO targets pre-mRNAs and regulates alternative splicing and 3’-end processing. Nucleic Acids Res 45(19):11356–11370. https://doi.org/10.1093/nar/gkx778
Tang C, Klukovich R, Peng H et al (2018) ALKBH5-dependent m6A demethylation controls splicing and stability of long 3’-UTR mRNAs in male germ cells. Proc Natl Acad Sci U S A 115(2):E325–E333. https://doi.org/10.1073/pnas.1717794115
Liao S, Sun H, Xu C (2018) YTH domain: a family of N(6)-methyladenosine (m(6)A) readers. Genomics Proteomics Bioinformatics 16(2):99–107. https://doi.org/10.1016/j.gpb.2018.04.002
Roundtree IA, Luo GZ, Zhang Z et al (2017) YTHDC1 mediates nuclear export of N(6)-methyladenosine methylated mRNAs. Elife 6(2050–084X (Electronic)). https://doi.org/10.7554/eLife.31311
Hsu PJ, Zhu Y, Ma H et al (2017) Ythdc2 is an N(6)-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27(9):1115–1127. https://doi.org/10.1038/cr.2017.99
Alarcon CR, Goodarzi H, Lee H et al (2015) HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell 162(6):1299–1308. https://doi.org/10.1016/j.cell.2015.08.011
Zhou KI, Shi H, Lyu R et al (2019) Regulation of co-transcriptional Pre-mRNA Splicing by m(6)A through the low-complexity protein hnRNPG. Mol Cell 76(1):70–81 e9. https://doi.org/10.1016/j.molcel.2019.07.005
Ries RJ, Zaccara S, Klein P et al (2019) m(6)A enhances the phase separation potential of mRNA. Nature 571(7765):424–428. https://doi.org/10.1038/s41586-019-1374-1
Li M, Zhao X, Wang W et al (2018) Ythdf2-mediated m(6)A mRNA clearance modulates neural development in mice. Genome Biol 19(1):69. https://doi.org/10.1186/s13059-018-1436-y
Li Z, Qian P, Shao W et al (2018) Suppression of m(6)A reader Ythdf2 promotes hematopoietic stem cell expansion. Cell Res 28(9):904–917. https://doi.org/10.1038/s41422-018-0072-0
Shi H, Wang X, Lu Z et al (2017) YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res 27(3):315–328. https://doi.org/10.1038/cr.2017.15
Martin GH, Park CY (2018) Meddling with METTLs in normal and leukemia stem cells. Cell Stem Cell 22(2):139–141. https://doi.org/10.1016/j.stem.2018.01.013
Zhang C, Chen Y, Sun B et al (2017) m(6)A modulates haematopoietic stem and progenitor cell specification. Nature 549(7671):273–276. https://doi.org/10.1038/nature23883
Yao QJ, Sang L, Lin M et al (2018) Mettl3-Mettl14 methyltransferase complex regulates the quiescence of adult hematopoietic stem cells. Cell Res 28(9):952–954. https://doi.org/10.1038/s41422-018-0062-2
Lee H, Bao S, Qian Y et al (2019) Stage-specific requirement for Mettl3-dependent m(6)A mRNA methylation during haematopoietic stem cell differentiation. Nat Cell Biol 21(6):700–709. https://doi.org/10.1038/s41556-019-0318-1
Vu LP, Pickering BF, Cheng Y et al (2017) The N(6)-methyladenosine (m(6)A)-forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 23(11):1369–1376. https://doi.org/10.1038/nm.4416
Gao Y, Vasic R, Song Y et al (2020) m(6)A Modification prevents formation of endogenous double-stranded RNAs and deleterious innate immune responses during hematopoietic development. Immunity 52(6):1007–1021 e8. https://doi.org/10.1016/j.immuni.2020.05.003
Weng H, Huang H, Wu H et al (2018) METTL14 Inhibits hematopoietic stem/progenitor differentiation and promotes leukemogenesis via mRNA m(6)A modification. Cell Stem Cell 22(2):191–205 e9. https://doi.org/10.1016/j.stem.2017.11.016
Mapperley C, van de Lagemaat LN, Lawson H et al (2021) The mRNA m6A reader YTHDF2 suppresses proinflammatory pathways and sustains hematopoietic stem cell function. J Exp Med 218(3). https://doi.org/10.1084/jem.20200829
Gu X, Zhang Y, Li D et al (2020) N6-methyladenosine demethylase FTO promotes M1 and M2 macrophage activation. Cell Signal 69(1873–3913 (Electronic)):109553. https://doi.org/10.1016/j.cellsig.2020.109553
Han D, Liu J, Chen C et al (2019) Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature 566(7743):270–274. https://doi.org/10.1038/s41586-019-0916-x
Li HB, Tong J, Zhu S et al (2017) m(6)A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature 548(7667):338–342. https://doi.org/10.1038/nature23450
Tong J, Cao G, Zhang T et al (2018) m(6)A mRNA methylation sustains Treg suppressive functions. Cell Res 28(2):253–256. https://doi.org/10.1038/cr.2018.7
Wang H, Hu X, Huang M et al (2019) Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat Commun 10(1):1898. https://doi.org/10.1038/s41467-019-09903-6
Chen H, Pan Y, Zhou Q et al (2022) METTL3 inhibits antitumor immunity by targeting m(6)A-BHLHE41-CXCL1/CXCR2 axis to promote colorectal cancer. Gastroenterology. https://doi.org/10.1053/j.gastro.2022.06.024
Cully M (2020) RNA methyltransferase inhibitor reduces AML. Nat Rev Drug Discov 19(8):510. https://doi.org/10.1038/d41573-020-00120-1
Li D, Liang J, Cheng C et al (2021) Identification of m6A-related lncRNAs associated with prognoses and immune responses in acute myeloid leukemia. Front Cell Dev Biol 9:770451 https://doi.org/10.3389/fcell.2021.770451
Han S, Qi J, Fang K et al (2022) Characterization of m6A regulator-mediated methylation modification patterns and tumor microenvironment infiltration in acute myeloid leukemia. Cancer Med 11(5):1413–1426. https://doi.org/10.1002/cam4.4531
Jia G, Fu Y, Zhao X et al (2011) N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat Chem Biol 7(12):885–887. https://doi.org/10.1038/nchembio.687
Olsen SN, Armstrong SA (2020) It’s not what you say but how you say it: targeting RNA methylation in AML. Mol Cell 78(6):996–998. https://doi.org/10.1016/j.molcel.2020.05.027
Li Z, Weng H, Su R et al (2017) FTO plays an oncogenic role in acute myeloid leukemia as a N(6)-methyladenosine RNA demethylase. Cancer Cell 31(1):127–141. https://doi.org/10.1016/j.ccell.2016.11.017
Su R, Dong L, Li Y et al (2020) Targeting FTO suppresses cancer stem cell maintenance and immune evasion. Cancer Cell 38(1):79–96 e11. https://doi.org/10.1016/j.ccell.2020.04.017
Huang Y, Su R, Sheng Y et al (2019) Small-molecule targeting of oncogenic FTO demethylase in acute myeloid leukemia. Cancer Cell 35(4):677–691 e10. https://doi.org/10.1016/j.ccell.2019.03.006
Sun K, Du Y, Hou Y et al (2021) Saikosaponin D exhibits anti-leukemic activity by targeting FTO/m(6)A signaling. Theranostics 11(12):5831–5846. https://doi.org/10.7150/thno.55574
Su R, Dong L, Li C et al (2018) R-2HG Exhibits anti-tumor activity by targeting FTO/m(6)A/MYC/CEBPA signaling. Cell 172(1–2):90–105 e23. https://doi.org/10.1016/j.cell.2017.11.031
Sulkowski PL, Corso CD, Robinson ND et al (2017) 2-Hydroxyglutarate produced by neomorphic IDH mutations suppresses homologous recombination and induces PARP inhibitor sensitivity. Sci Transl Med 9(375). https://doi.org/10.1126/scitranslmed.aal2463
Clark O, Yen K, Mellinghoff IK (2016) Molecular pathways: isocitrate dehydrogenase mutations in cancer. Clin Cancer Res 22(8):1837–1842. https://doi.org/10.1158/1078-0432.CCR-13-1333
Qing Y, Dong L, Gao L et al (2021) R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m(6)A/PFKP/LDHB axis. Mol Cell 81(5):922–939 e9. https://doi.org/10.1016/j.molcel.2020.12.026
Ye D, Ma S, Xiong Y et al (2013) R-2-hydroxyglutarate as the key effector of IDH mutations promoting oncogenesis. Cancer Cell 23(3):274–276. https://doi.org/10.1016/j.ccr.2013.03.005
(2018) R-2HG Targets FTO to increase m(6)A levels and suppress tumor growth. Cancer Discov 8(2):137. https://doi.org/10.1158/2159-8290.CD-RW2017-240
Yang S, Wei J, Cui YH et al (2019) m(6)A mRNA demethylase FTO regulates melanoma tumorigenicity and response to anti-PD-1 blockade. Nat Commun 10(1):2782. https://doi.org/10.1038/s41467-019-10669-0
Liu Y, Liang G, Xu H et al (2021) Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab 33(6):1221–1233 e11. https://doi.org/10.1016/j.cmet.2021.04.001
Zhang S, Zhao BS, Zhou A, et al (2017) m(6)A demethylase ALKBH5 maintains Tumorigenicity of glioblastoma stem-like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31(4):591–606 e6. https://doi.org/10.1016/j.ccell.2017.02.013
Kwok CT, Marshall AD, Rasko JE et al (2017) Genetic alterations of m(6)A regulators predict poorer survival in acute myeloid leukemia. J Hematol Oncol 10(1):39. https://doi.org/10.1186/s13045-017-0410-6
Deng X, Su R, Weng H et al (2018) RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res 28(5):507–517. https://doi.org/10.1038/s41422-018-0034-6
Cancer Genome Atlas Research N, Ley TJ, Miller C et al (2013) Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N Engl J Med 368(22):2059–74. https://doi.org/10.1056/NEJMoa1301689
Shen C, Sheng Y, Zhu AC et al (2020) RNA Demethylase ALKBH5 selectively promotes tumorigenesis and cancer stem cell self-renewal in acute myeloid leukemia. Cell Stem Cell 27(1):64–80 e9. https://doi.org/10.1016/j.stem.2020.04.009
Wang J, Li Y, Wang P et al (2020) Leukemogenic chromatin alterations promote AML leukemia stem cells via a KDM4C-ALKBH5-AXL signaling axis. Cell Stem Cell 27(1):81–97 e8. https://doi.org/10.1016/j.stem.2020.04.001
Zhang L, Su X (2022) Bioactive peptide inhibits acute myeloid leukemia cell proliferation by downregulating ALKBH5-mediated m(6)A demethylation of EIF4EBP1 and MLST8 mRNA. Cell Oncol (Dordr) 45(3):355–365. https://doi.org/10.1007/s13402-022-00666-9
Zhu C, Wei Y, Wei X (2019) AXL receptor tyrosine kinase as a promising anti-cancer approach: functions, molecular mechanisms and clinical applications. Mol Cancer 18(1):153. https://doi.org/10.1186/s12943-019-1090-3
Graham DK, DeRyckere D, Davies KD et al (2014) The TAM family: phosphatidylserine sensing receptor tyrosine kinases gone awry in cancer. Nat Rev Cancer 14(12):769–785. https://doi.org/10.1038/nrc3847
Hong CC, Lay JD, Huang JS et al (2008) Receptor tyrosine kinase AXL is induced by chemotherapy drugs and overexpression of AXL confers drug resistance in acute myeloid leukemia. Cancer Lett 268(2):314–324. https://doi.org/10.1016/j.canlet.2008.04.017
Yan F, Al-Kali A, Zhang Z et al (2018) A dynamic N(6)-methyladenosine methylome regulates intrinsic and acquired resistance to tyrosine kinase inhibitors. Cell Res 28(11):1062–1076. https://doi.org/10.1038/s41422-018-0097-4
Zhao BS, Roundtree IA, He C (2017) Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol 18(1):31–42. https://doi.org/10.1038/nrm.2016.132
Choe J, Lin S, Zhang W et al (2018) mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature 561(7724):556–560. https://doi.org/10.1038/s41586-018-0538-8
Barbieri I, Tzelepis K, Pandolfini L et al (2017) Promoter-bound METTL3 maintains myeloid leukaemia by m(6)A-dependent translation control. Nature 552(7683):126–131. https://doi.org/10.1038/nature24678
Wiedmer L, Eberle SA, Bedi RK et al (2019) A reader-based assay for m(6)A writers and erasers. Anal Chem 91(4):3078–3084. https://doi.org/10.1021/acs.analchem.8b05500
Bedi RK, Huang D, Eberle SA et al (2020) Small-molecule inhibitors of METTL3, the major human epitranscriptomic writer. ChemMedChem 15(9):744–748. https://doi.org/10.1002/cmdc.202000011
Moroz-Omori EV, Huang D, Kumar Bedi R et al (2021) METTL3 inhibitors for epitranscriptomic modulation of cellular processes. ChemMedChem 16(19):3035–3043. https://doi.org/10.1002/cmdc.202100291
Yankova E, Blackaby W, Albertella M et al (2021) Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature 593(7860):597–601. https://doi.org/10.1038/s41586-021-03536-w
Ni Z, Sun P, Zheng J et al (2022) JNK signaling promotes bladder cancer immune escape by regulating METTL3-mediated m6A modification of PD-L1 mRNA. Cancer Res 82(9):1789–1802. https://doi.org/10.1158/0008-5472.CAN-21-1323
Little NA, Hastie ND, Davies RC (2000) Identification of WTAP, a novel Wilms’ tumour 1-associating protein. Hum Mol Genet 9(15):2231–2239. https://doi.org/10.1093/oxfordjournals.hmg.a018914
Bansal H, Yihua Q, Iyer SP et al (2014) WTAP is a novel oncogenic protein in acute myeloid leukemia. Leukemia 28(5):1171–1174. https://doi.org/10.1038/leu.2014.16
Sorci M, Ianniello Z, Cruciani S et al (2018) METTL3 regulates WTAP protein homeostasis. Cell Death Dis 9(8):796. https://doi.org/10.1038/s41419-018-0843-z
Zhang L, Tran NT, Su H et al. (2015) Cross-talk between PRMT1-mediated methylation and ubiquitylation on RBM15 controls RNA splicing. Elife 4. https://doi.org/10.7554/eLife.07938
Ma X, Renda MJ, Wang L et al (2007) Rbm15 modulates notch-induced transcriptional activation and affects myeloid differentiation. Mol Cell Biol 27(8):3056–3064. https://doi.org/10.1128/MCB.01339-06
Ma Z, Morris SW, Valentine V et al (2001) Fusion of two novel genes, RBM15 and MKL1, in the t(1;22)(p13;q13) of acute megakaryoblastic leukemia. Nat Genet 28(3):220–221. https://doi.org/10.1038/90054
Tran NT, Su H, Khodadadi-Jamayran A et al (2016) The AS-RBM15 lncRNA enhances RBM15 protein translation during megakaryocyte differentiation. EMBO Rep 17(6):887–900. https://doi.org/10.15252/embr.201541970
Sheng Y, Wei J, Yu F et al (2021) A critical role of nuclear m6A reader YTHDC1 in leukemogenesis by regulating MCM complex-mediated DNA replication. Blood 138(26):2838–2852. https://doi.org/10.1182/blood.2021011707
Paris J, Morgan M, Campos J et al (2019) Targeting the RNA m(6)A reader YTHDF2 Selectively compromises cancer stem cells in acute myeloid leukemia. Cell Stem Cell 25(1):137–148 e6. https://doi.org/10.1016/j.stem.2019.03.021
Chen Z, Shao YL, Wang LL et al (2021) YTHDF2 is a potential target of AML1/ETO-HIF1alpha loop-mediated cell proliferation in t(8;21) AML. Oncogene 40(22):3786–3798. https://doi.org/10.1038/s41388-021-01818-1
Feng M, Xie X, Han G et al (2021) YBX1 is required for maintaining myeloid leukemia cell survival by regulating BCL2 stability in an m6A-dependent manner. Blood 138(1):71–85. https://doi.org/10.1182/blood.2020009676
Zhang N, Shen Y, Li H et al (2022) The m6A reader IGF2BP3 promotes acute myeloid leukemia progression by enhancing RCC2 stability. Exp Mol Med 54(2):194–205. https://doi.org/10.1038/s12276-022-00735-x
Ishikawa Y, Kawashima N, Atsuta Y et al (2020) Prospective evaluation of prognostic impact of KIT mutations on acute myeloid leukemia with RUNX1-RUNX1T1 and CBFB-MYH11. Blood Adv 4(1):66–75. https://doi.org/10.1182/bloodadvances.2019000709
Lv K, Ren JG, Han X et al (2021) Depalmitoylation rewires FLT3-ITD signaling and exacerbates leukemia progression. Blood 138(22):2244–2255. https://doi.org/10.1182/blood.2021011582
Thol F, Ganser A (2020) Treatment of relapsed acute myeloid leukemia. Curr Treat Options Oncol 21(8):66. https://doi.org/10.1007/s11864-020-00765-5
Jin D, Guo J, Wu Y et al (2019) m(6)A mRNA methylation initiated by METTL3 directly promotes YAP translation and increases YAP activity by regulating the MALAT1-miR-1914-3p-YAP axis to induce NSCLC drug resistance and metastasis. J Hematol Oncol 12(1):135. https://doi.org/10.1186/s13045-019-0830-6
Taketo K, Konno M, Asai A et al (2018) The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol 52(2):621–629. https://doi.org/10.3892/ijo.2017.4219
Zhang Y, Kang M, Zhang B et al (2019) m(6)A modification-mediated CBX8 induction regulates stemness and chemosensitivity of colon cancer via upregulation of LGR5. Mol Cancer 18(1):185. https://doi.org/10.1186/s12943-019-1116-x
Funding
This work was funded by the National Natural Science Foundation of China [U2001224], the Frontier Research Program of Guangzhou Regenerative Medicine and Health Guangdong Laboratory [2018GZR110105014], and the Guangdong Basic and Applied Basic Research Foundation [2021A1515110974].
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Human and animal rights and informed consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hu, R., Liao, P., Xu, B. et al. N6-methyladenosine RNA modifications: a potential therapeutic target for AML. Ann Hematol (2023). https://doi.org/10.1007/s00277-023-05302-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00277-023-05302-6