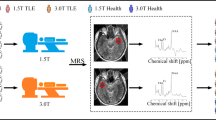
Abstract
Purpose
Precise lateralizing the epileptogenic zone in patients with drug-resistant mesial temporal lobe epilepsy (mTLE) remains challenging, particularly when routine MRI scans are inconclusive (MRI-negative). This study aimed to investigate the synergy of fast, high-resolution, whole-brain MRSI in conjunction with simultaneous [18F]FDG PET for the lateralization of mTLE.
Methods
Forty-eight drug-resistant mTLE patients (M/F 31/17, age 12–58) underwent MRSI and [18F]FDG PET on a hybrid PET/MR scanner. Lateralization of mTLE was evaluated by visual inspection and statistical classifiers of metabolic mappings against routine MRI. Additionally, this study explored how disease status influences the associations between altered N-acetyl aspartate (NAA) and FDG uptake using hierarchical moderated multiple regression.
Results
The high-resolution whole-brain MRSI data offers metabolite maps at comparable resolution to [18F]FDG PET. Visual examinations of combined MRSI and [18F]FDG PET showed an mTLE lateralization accuracy rate of 91.7% in a 48-patient cohort, surpassing routine MRI (52.1%). Notably, out of 23 MRI-negative mTLE, combined MRSI and [18F]FDG PET helped detect 19 cases. Logistical regression models combining hippocampal NAA level and FDG uptake improved lateralization performance (AUC=0.856), while further incorporating extrahippocampal regions such as amygdala, thalamus, and superior temporal gyrus increased the AUC to 0.939. Concurrent MRSI/PET revealed a moderating influence of disease duration and hippocampal atrophy on the association between hippocampal NAA and glucose uptake, providing significant new insights into the disease’s trajectory.
Conclusion
This paper reports the first metabolic imaging study using simultaneous high-resolution MRSI and [18F]FDG PET, which help visualize MRI-unidentifiable lesions and may thus advance diagnostic tools and management strategies for drug-resistant mTLE.







Similar content being viewed by others
Data Availability
The data that support the findings of this study are available upon request from the corresponding author, J.L.
References
Thijs RD, Surges R, O’Brien TJ, Sander JW. Epilepsy in adults. Lancet. 2019;393:689–701. https://doi.org/10.1016/s0140-6736(18)32596-0.
Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug-resistant temporal lobe epilepsy: a randomized trial. Jama. 2012;307:922–30. https://doi.org/10.1001/jama.2012.220.
Thom M. Review: Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathol Appl Neurobiol. 2014;40:520–43. https://doi.org/10.1111/nan.12150.
Blümcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia. 2013;54:1315–29. https://doi.org/10.1111/epi.12220.
Muhlhofer W, Tan YL, Mueller SG, Knowlton R. MRI-negative temporal lobe epilepsy-what do we know? Epilepsia. 2017;58:727–42. https://doi.org/10.1111/epi.13699.
Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–8. https://doi.org/10.1016/j.eplepsyres.2010.02.007.
Duncan JS, Winston GP, Koepp MJ, Ourselin S. Brain imaging in the assessment for epilepsy surgery. The Lancet Neurology. 2016;15:420–33. https://doi.org/10.1016/s1474-4422(15)00383-x.
Lagarde S, Boucekine M, McGonigal A, Carron R, Scavarda D, Trebuchon A, et al. Relationship between PET metabolism and SEEG epileptogenicity in focal lesional epilepsy. Eur J Nucl Med Mol Imaging. 2020;47:3130–42. https://doi.org/10.1007/s00259-020-04791-1.
Lee EM, Park GY, Im KC, Kim ST, Woo CW, Chung JH, et al. Changes in glucose metabolism and metabolites during the epileptogenic process in the lithium-pilocarpine model of epilepsy. Epilepsia. 2012;53:860–9. https://doi.org/10.1111/j.1528-1167.2012.03432.x.
Filibian M, Frasca A, Maggioni D, Micotti E, Vezzani A, Ravizza T. In vivo imaging of glia activation using 1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia. 2012;53:1907–16. https://doi.org/10.1111/j.1528-1167.2012.03685.x.
Reddy SD, Younus I, Sridhar V, Reddy DS. Neuroimaging biomarkers of experimental epileptogenesis and refractory epilepsy. Int J Mol Sci. 2019;20(1):220. https://doi.org/10.3390/ijms20010220.
Doelken MT, Stefan H, Pauli E, Stadlbauer A, Struffert T, Engelhorn T, et al. (1)H-MRS profile in MRI positive- versus MRI negative patients with temporal lobe epilepsy. Seizure. 2008;17:490–7. https://doi.org/10.1016/j.seizure.2008.01.008.
Rho JM, Boison D. The metabolic basis of epilepsy. Nat Rev Neurol. 2022;18:333–47. https://doi.org/10.1038/s41582-022-00651-8.
Carne RP, O’Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127:2276–85. https://doi.org/10.1093/brain/awh257.
LoPinto-Khoury C, Sperling MR, Skidmore C, Nei M, Evans J, Sharan A, et al. Surgical outcome in PET-positive, MRI-negative patients with temporal lobe epilepsy. Epilepsia. 2012;53:342–8. https://doi.org/10.1111/j.1528-1167.2011.03359.x.
Niu N, Xing H, Wu M, Ma Y, Liu Y, Ba J, et al. Performance of PET imaging for the localization of epileptogenic zone in patients with epilepsy: a meta-analysis. Eur Radiol. 2021. https://doi.org/10.1007/s00330-020-07645-4.
Oz G, Alger JR, Barker PB, Bartha R, Bizzi A, Boesch C, et al. Clinical proton MR spectroscopy in central nervous system disorders. Radiology. 2014;270:658–79. https://doi.org/10.1148/radiol.13130531.
Pan JW, Kuzniecky RI. Utility of magnetic resonance spectroscopic imaging for human epilepsy. Quant Imaging Med Surg. 2015;5:313–22. https://doi.org/10.3978/j.issn.2223-4292.2015.01.03.
Pan JW, Williamson A, Cavus I, Hetherington HP, Zaveri H, Petroff OA, et al. Neurometabolism in human epilepsy. Epilepsia. 2008;49(Suppl 3):31–41. https://doi.org/10.1111/j.1528-1167.2008.01508.x.
Fernández-Vega N, Ramos-Rodriguez JR, Alfaro F, Barbancho M, García-Casares N. Usefulness of magnetic resonance spectroscopy in mesial temporal sclerosis: a systematic review. Neuroradiology. 2021;63:1395–405. https://doi.org/10.1007/s00234-021-02704-z.
Vermathen P, Ende G, Laxer KD, Knowlton RC, Matson GB, Weiner MW. Hippocampal N-acetylaspartate in neocortical epilepsy and mesial temporal lobe epilepsy. Ann Neurol. 1997;42:194–9. https://doi.org/10.1002/ana.410420210.
Petroff OA, Errante LD, Kim JH, Spencer DD. N-acetyl-aspartate, total creatine, and myo-inositol in the epileptogenic human hippocampus. Neurology. 2003;60:1646–51. https://doi.org/10.1212/01.wnl.0000068020.85450.8b.
Cendes F, Andermann F, Preul MC, Arnold DL. Lateralization of temporal lobe epilepsy based on regional metabolic abnormalities in proton magnetic resonance spectroscopic images. Ann Neurol. 1994;35:211–6. https://doi.org/10.1002/ana.410350213.
Connelly A, Van Paesschen W, Porter DA, Johnson CL, Duncan JS, Gadian DG. Proton magnetic resonance spectroscopy in MRI-negative temporal lobe epilepsy. Neurology. 1998;51:61–6. https://doi.org/10.1212/wnl.51.1.61.
Hetherington HP, Kuzniecky RI, Vives K, Devinsky O, Pacia S, Luciano D, et al. A subcortical network of dysfunction in TLE measured by magnetic resonance spectroscopy. Neurology. 2007;69:2256–65. https://doi.org/10.1212/01.wnl.0000286945.21270.6d.
Pan JW, Duckrow RB, Gerrard J, Ong C, Hirsch LJ, Resor SR Jr, et al. 7T MR spectroscopic imaging in the localization of surgical epilepsy. Epilepsia. 2013;54:1668–78. https://doi.org/10.1111/epi.12322.
Mueller SG, Laxer KD, Cashdollar N, Flenniken DL, Matson GB, Weiner MW. Identification of abnormal neuronal metabolism outside the seizure focus in temporal lobe epilepsy. Epilepsia. 2004;45:355–66. https://doi.org/10.1111/j.0013-9580.2004.27603.x.
Tan Q, Sun H, Wang W, Wu X, Hao N, Su X, et al. Quantitative MR spectroscopy reveals metabolic changes in the dorsolateral prefrontal cortex of patients with temporal lobe epilepsy. Eur Radiol. 2018;28:4496–503. https://doi.org/10.1007/s00330-018-5443-x.
Capizzano AA, Vermathen P, Laxer KD, Matson GB, Maudsley AA, Soher BJ, et al. Multisection proton MR spectroscopy for mesial temporal lobe epilepsy. AJNR Am J Neuroradiol. 2002;23:1359–68.
Spencer DD, Gerrard JL, Zaveri HP. The roles of surgery and technology in understanding focal epilepsy and its comorbidities. Lancet Neurol. 2018;17:373–82. https://doi.org/10.1016/s1474-4422(18)30031-0.
Zijlmans M, Zweiphenning W, van Klink N. Changing concepts in presurgical assessment for epilepsy surgery. Nat Rev Neurol. 2019;15:594–606. https://doi.org/10.1038/s41582-019-0224-y.
van der Veen DR, Shao J, Chapman S, Leevy WM, Duffield GE. A 24-hour temporal profile of in vivo brain and heart pet imaging reveals a nocturnal peak in brain 18F-fluorodeoxyglucose uptake. PLoS One. 2012;7:e31792. https://doi.org/10.1371/journal.pone.0031792.
Soreni N, Noseworthy MD, Cormier T, Oakden WK, Bells S, Schachar R. Intraindividual variability of striatal (1)H-MRS brain metabolite measurements at 3 T. Magn Reson Imaging. 2006;24:187–94. https://doi.org/10.1016/j.mri.2005.10.027.
Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med. 2009;50(Suppl 1):11s–20s. https://doi.org/10.2967/jnumed.108.057182.
Lam F, Liang Z-PA. subspace approach to high-resolution spectroscopic imaging. Magn Reson Med. 2014;71:1349–57. https://doi.org/10.1002/mrm.25168.
Ma C, Lam F, Ning Q, Johnson CL, Liang Z-P. High-resolution (1) H-MRSI of the brain using short-TE SPICE. Magn Reson Med. 2017;77:467–79. https://doi.org/10.1002/mrm.26130.
Li Y, Lam F, Clifford B, Liang Z-PA. subspace approach to spectral quantification for MR spectroscopic imaging. IEEE Trans Biomed Eng. 2017;64:2486–9. https://doi.org/10.1109/tbme.2017.2741922.
Guo R, Zhao Y, Li Y, Li Y, Liang Z-P. Simultaneous metabolic and functional imaging of the brain using SPICE. Magn Reson Med. 2019;82:1993–2002. https://doi.org/10.1002/mrm.27865.
Peng X, Lam F, Li Y, Clifford B, Liang Z-P. Simultaneous QSM and metabolic imaging of the brain using SPICE. Magn Reson Med. 2018;79:13–21. https://doi.org/10.1002/mrm.26972.
Li Y, Wang T, Zhang T, Lin Z, Li Y, Guo R, et al. Fast high-resolution metabolic imaging of acute stroke with 3D magnetic resonance spectroscopy. Brain. 2020;143:3225–33. https://doi.org/10.1093/brain/awaa264.
Lin Z, Meng Z, Wang T, Guo R, Zhao Y, Li Y, et al. Predicting the onset of ischemic stroke with fast high-resolution 3D MR Spectroscopic Imaging. J Magn Reson Imaging. 2023. https://doi.org/10.1002/jmri.28596.
Guo R, Ma C, Li Y, Zhao Y, Wang T, Li Y, et al. High-resolution label-free molecular imaging of brain tumor. Annu Int Conf IEEE Eng Med Biol Soc. 2021;2021:3049–52. https://doi.org/10.1109/embc46164.2021.9630623.
Lam F, Ma C, Clifford B, Johnson CL, Liang Z-P. High-resolution (1) H-MRSI of the brain using SPICE: data acquisition and image reconstruction. Magn Reson Med. 2016;76:1059–70. https://doi.org/10.1002/mrm.26019.
Liang ZP Spatiotemporal imagingwith partially separable functions. 2007 4th IEEE international symposium on biomedical imaging: from nano to macro: IEEE; 2007; 988-91.
Lin A, Andronesi O, Bogner W, Choi IY, Coello E, Cudalbu C, et al. Minimum reporting standards for in vivo magnetic resonance spectroscopy (MRSinMRS): experts’ consensus recommendations. NMR Biomed. 2021;34:e4484. https://doi.org/10.1002/nbm.4484.
Koesters T, Friedman KP, Fenchel M, Zhan Y, Hermosillo G, Babb J, et al. Dixon sequence with superimposed model-based bone compartment provides highly accurate PET/MR attenuation correction of the brain. J Nucl Med. 2016;57:918–24. https://doi.org/10.2967/jnumed.115.166967.
Presotto L, Ballarini T, Caminiti SP, Bettinardi V, Gianolli L, Perani D. Validation of (18)F-FDG-PET single-subject optimized SPM procedure with different PET scanners. Neuroinformatics. 2017;15:151–63. https://doi.org/10.1007/s12021-016-9322-9.
Spencer SS. Neural networks in human epilepsy: evidence of and implications for treatment. Epilepsia. 2002;43:219–27. https://doi.org/10.1046/j.1528-1157.2002.26901.x.
Gu Q, Li Z, Han J. Generalized fisher score for feature selection. arXiv preprint arXiv:12023725. 2012.
Aguinis H, Gottfredson RK. Best-practice recommendations for estimating interaction effects using moderated multiple regression. J Organ Behav. 2010;31:776–86. https://doi.org/10.1002/job.686.
Whisman MA, McClelland GH. Designing, testing, and interpreting interactions and moderator effects in family research. J Fam Psychol. 2005;19:111–20. https://doi.org/10.1037/0893-3200.19.1.111.
Gleichgerrcht E, Munsell BC, Alhusaini S, Alvim MKM, Bargalló N, Bender B, et al. Artificial intelligence for classification of temporal lobe epilepsy with ROI-level MRI data: a worldwide ENIGMA-Epilepsy study. Neuroimage Clin. 2021;31:102765. https://doi.org/10.1016/j.nicl.2021.102765.
Pan JW, Spencer DD, Kuzniecky R, Duckrow RB, Hetherington H, Spencer SS. Metabolic networks in epilepsy by MR spectroscopic imaging. Acta Neurol Scand. 2012;126:411–20. https://doi.org/10.1111/j.1600-0404.2012.01665.x.
Kirov II, Kuzniecky R, Hetherington HP, Soher BJ, Davitz MS, Babb JS, et al. Whole brain neuronal abnormalities in focal epilepsy quantified with proton MR spectroscopy. Epilepsy Res. 2018;139:85–91. https://doi.org/10.1016/j.eplepsyres.2017.11.017.
Amorim-Leite R, Remick M, Welch W, Abel TJ. History of the Network Approach in Epilepsy Surgery. Neurosurg Clin N Am. 2020;31:301–8. https://doi.org/10.1016/j.nec.2020.03.011.
Bartolomei F, Chauvel P, Wendling F. Epileptogenicity of brain structures in human temporal lobe epilepsy: a quantified study from intracerebral EEG. Brain. 2008;131:1818–30. https://doi.org/10.1093/brain/awn111.
Guo Y, Gao F, Wang S, Ding Y, Zhang H, Wang J, et al. In vivo mapping of temporospatial changes in glucose utilization in rat brain during epileptogenesis: an 18F-fluorodeoxyglucose-small animal positron emission tomography study. Neuroscience. 2009;162:972–9. https://doi.org/10.1016/j.neuroscience.2009.05.041.
Aparicio J, Carreño M, Bargalló N, Setoain X, Rubí S, Rumià J, et al. Combined (18)F-FDG-PET and diffusion tensor imaging in mesial temporal lobe epilepsy with hippocampal sclerosis. Neuroimage Clin. 2016;12:976–89. https://doi.org/10.1016/j.nicl.2016.05.002.
Scanlon C, Mueller SG, Cheong I, Hartig M, Weiner MW, Laxer KD. Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. J Neurol. 2013;260:2320–9. https://doi.org/10.1007/s00415-013-6974-3.
Riederer F, Lanzenberger R, Kaya M, Prayer D, Serles W, Baumgartner C. Network atrophy in temporal lobe epilepsy: a voxel-based morphometry study. Neurology. 2008;71:419–25. https://doi.org/10.1212/01.wnl.0000324264.96100.e0.
Kikuchi K, Togao O, Yamashita K, Momosaka D, Nakayama T, Kitamura Y, et al. Diagnostic accuracy for the epileptogenic zone detection in focal epilepsy could be higher in FDG-PET/MRI than in FDG-PET/CT. Eur Radiol. 2021;31:2915–22. https://doi.org/10.1007/s00330-020-07389-1.
Guo K, Cui B, Shang K, Hou Y, Fan X, Yang H, et al. Assessment of localization accuracy and postsurgical prediction of simultaneous (18)F-FDG PET/MRI in refractory epilepsy patients. Eur Radiol. 2021. https://doi.org/10.1007/s00330-021-07738-8.
Guo K, Wang J, Wang Z, Wang Y, Cui B, Zhao G, et al. Morphometric analysis program and quantitative positron emission tomography in presurgical localization in MRI-negative epilepsies: a simultaneous PET/MRI study. Eur J Nucl Med Mol Imaging. 2022;49:1930–8. https://doi.org/10.1007/s00259-021-05657-w.
Li Y, Zhang T, Feng J, Qian S, Wu S, Zhou R, et al. Processing speed dysfunction is associated with functional corticostriatal circuit alterations in childhood epilepsy with centrotemporal spikes: a PET and fMRI study. Eur J Nucl Med Mol Imaging. 2022;49:3186–96. https://doi.org/10.1007/s00259-022-05740-w.
Yuan S, Huang H, Cai B, Li J, Zhang M, Luo J. Altered metabolic-functional coupling in the epileptogenic network could predict surgical outcomes of mesial temporal lobe epilepsy. Front Neurosci. 2023;17:1165982. https://doi.org/10.3389/fnins.2023.1165982.
Zhang M, Huang H, Liu W, Tang L, Li Q, Wang J, et al. Combined quantitative T2 mapping and [(18)F]FDG PET could improve lateralization of mesial temporal lobe epilepsy. Eur Radiol. 2022;32:6108–17. https://doi.org/10.1007/s00330-022-08707-5.
Shang K, Wang J, Fan X, Cui B, Ma J, Yang H, et al. Clinical value of hybrid TOF-PET/MR imaging-based multiparametric imaging in localizing seizure focus in patients with MRI-Negative temporal lobe epilepsy. AJNR Am J Neuroradiol. 2018;39:1791–8. https://doi.org/10.3174/ajnr.A5814.
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol. 2007;81:89–131. https://doi.org/10.1016/j.pneurobio.2006.12.003.
Moffett JR, Arun P, Ariyannur PS, Namboodiri AM. N-Acetylaspartate reductions in brain injury: impact on post-injury neuroenergetics, lipid synthesis, and protein acetylation. Front Neuroenergetics. 2013;5:11. https://doi.org/10.3389/fnene.2013.00011.
Clark JF, Doepke A, Filosa JA, Wardle RL, Lu A, Meeker TJ, et al. N-acetylaspartate as a reservoir for glutamate. Med Hypotheses. 2006;67:506–12. https://doi.org/10.1016/j.mehy.2006.02.047.
Zhang L, Guo Y, Hu H, Wang J, Liu Z, Gao F. FDG-PET and NeuN-GFAP immunohistochemistry of hippocampus at different phases of the pilocarpine model of temporal lobe epilepsy. Int J Med Sci. 2015;12:288–94. https://doi.org/10.7150/ijms.10527.
Funding
The study was partially supported by the National Natural Science Foundation of China (No. 62101321, and No. 82372073), Shanghai Municipal Health Commission (No. 202240031), and Shanghai Municipal Key Clinical Specialty (shslczdzk03403).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Ruijin hospital (Approved No. 2016-128).
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Zhang, M., Zhao, Y. et al. Simultaneous high-resolution whole-brain MR spectroscopy and [18F]FDG PET for temporal lobe epilepsy. Eur J Nucl Med Mol Imaging 51, 721–733 (2024). https://doi.org/10.1007/s00259-023-06465-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-023-06465-0