Abstract
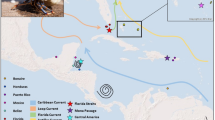
Phylogeography provides insights into how historical and contemporary processes influence the genetic structure and gene flow in marine organisms around the globe. In benthic marine invertebrates, a species’ reproductive strategy can strongly impact phylogeographic patterns and distribution, with some direct-developing (non-planktonic) dispersers demonstrating strong genetic structure but also broad geographic spread. While seemingly paradoxical, past work has shown ovoviviparous species, like Littorina saxatilis, can be more successful colonizers of remote locations than species with planktonic larvae, like L. littorea. Both Littorina species overlap in much of their North Atlantic ranges but have different colonization histories: L. saxatilis is native on both North Atlantic coasts and islands, and L. littorea is native to the eastern Atlantic but introduced to the west. Using an extensive mitochondrial dataset (1236 sequences; 85 sites), we examined how their opposing reproductive strategies correspond to their distributions and phylogeographies. Littorina saxatilis exhibited a heterogeneous genetic structure reflecting post-glacial recolonization from multiple refugial sites, while L. littorea had a homogeneous structure with a post-glacial history characterized by recolonization from one main refugial area in the northeast Atlantic. Further, haplotype diversity was significantly depressed in northwest Atlantic L. littorea populations, signifying a strong bottleneck characteristic of a human-mediated introduction. In contrast, haplotype diversity in L. saxatilis was similar between the two regions, demonstrating long-term history on both coasts. Thus, our study suggests contrasting life-history characteristics were a major structuring force in the phylogeographic patterns of these related species following large-scale disturbances (natural and anthropogenic) that compel contraction and redistribution over large areas.



Similar content being viewed by others
Availability of data and material (data transparency)
All data are available in GENBANK (https://www.ncbi.nlm.nih.gov/genbank/). Compilation of data herein are provided as tables, figures, or appendices in the manuscript.
Code availability
There was no code used in the manuscript.
References
Arndt A, Smith MJ (1998) Genetic diversity and population structure in two species of sea cucumber: differing patterns according to mode of development. Mol Ecol 7:1053–1064
Avise JC (2000) Phylogeography: the history and formation of species. Harvard University Press, Harvard
Ayre DJ, Minchinton TE, Perrin C (2009) Does life history predict past and current connectivity for rocky intertidal invertebrates across a marine biogeographic barrier? Mol Ecol 18(9):1887–1903
Blakeslee AMH, Byers JE, Lesser MP (2008) Solving cryptogenic histories using host and parasite molecular genetics: the resolution of Littorina littorea’s North American origin. Mol Ecol 17:3684–3696
Blakeslee AM, Altman I, Miller AW, Byers JE, Hamer CE, Ruiz GM (2012) Parasites and invasions: a biogeographic examination of parasites and hosts in native and introduced ranges. J Biogeogr 39(3):609–622
Blakeslee AM, Fowler AE, Couture JL, Grosholz ED, Ruiz GM, Miller AW (2016) Vector management reduces marine organisms transferred with live saltwater bait. Manag Biol Invasions 7(4):389–398
Bohonak AJ (1999) Dispersal, gene flow, and population structure. Q Rev Biol 74(1):21–45
Bowen BW, Gaither MR, DiBattista JD, Iacchei M, Andrews KR, Grant WS, Toonen RJ, Briggs JC (2016) Comparative phylogeography of the ocean planet. Proc Natl Acad Sci 113:7962–7969
Brawley SH, Coyer JA, Blakeslee AMH, Olsen JL, Hoarau G, Johnson LE, Byers JE, Stam WT (2009) Historical invasions of the intertidal zone of Atlantic North America associated with distinctive patterns of trade and emigration. Proc Natl Acad Sci USA 106:8239–8244
Byers JE, Pringle JM (2006) Going against the flow: retention, range limits and invasions in advective environments. Mar Ecol Prog Ser 313:27–41
Carlton JT (1982) The historical biogeography of Littorina littorea on the Atlantic coast of North America, and implications for the interpretation of the structure of New England intertidal communities. Malacol Rev 15:146
Carlton JT, Cohen AN (1998) Periwinkle’s progress: the Atlantic snail Littorina saxatilis (Mollusca: Gastropoda) establishes a colony on Pacific shores. Veliger 41:333–338
Chang AL, Blakeslee AMH, Miller AW, Ruiz GM (2011) Establishment failure in biological invasions: a case history of Littorina littorea in California, USA. PLoS ONE 6:e16035
Clement M, Posada D, Crandall KA (2000) TCS: a computer program to estimate gene genealogies. Mol Ecol 9:1657–1659
Collin R (2001) The effects of mode of development on phylogeography and population structure of North Atlantic Crepidula (Gastropoda: Calyptraeidae). Mol Ecol 10:2249–2262
Crandall ED, Frey MA, Grosberg RK, Barber PH (2008) Contrasting demographic history and phylogeographical patterns in two Indo–Pacific gastropods. Mol Ecol 17(2):611–626
Darling JA, Carlton JT (2018) A framework for understanding marine cosmopolitanism in the Anthropocene. Front Mar Sci 22(5):293
Doellman MM, Trussell GC, Grahame JW, Vollmer SV (2011) Phylogeographic analysis reveals a deep lineage split within North Atlantic Littorina saxatilis. Proc R Soc B: Biol Sci 278(1722):3175–3183
Excoffier L, Laval G, Schneider S (2005) Arlequin ver 3.0: an integrated software package for population genetics data analysis. Evol Bioinform Online 1:47–50
Foltz DW (2003) Invertebrate species with nonpelagic larvae have elevated levels of nonsynonymous substitutions and reduced nucleotide diversities. J Mol Evol 57:607–612
Foltz DW, Hrincevich AW, Rocha-Olivares A (2004) Apparent selection intensity for the cytochrome oxidase subunit I gene varies with mode of reproduction in echinoderms. Genetica 122:115–125
Fowler AE, Blakeslee AM, Canning-Clode J, Repetto MF, Phillip AM, Carlton JT, Moser FC, Ruiz GM, Miller AW (2016) Opening Pandora’s bait box: a potent vector for biological invasions of live marine species. Divers Distrib 22(1):30–42
Fraser CI, Nikula R, Waters JM (2011) Oceanic rafting by a coastal community. Proc R Soc B 278:649–655
Galtier N, Nabholz B, Glémin S, Hurst GDD (2009) Mitochondrial DNA as a marker of molecular diversity: a reappraisal. Mol Ecol 18:4541–4550
Geller JB, Darling JA, Carlton JT (2010) Genetic perspectives on marine biological invasions. Annu Rev Mar Sci 2:367–393
Harley CD, Anderson KM, Lebreton CAM, MacKay A, Ayala-Díaz M, Chong SL, Pond LM, Maddison JHA, Hung BH, Iversen SL, Wong DC (2013) The introduction of Littorina littorea to British Columbia, Canada: potential impacts and the importance of biotic resistance by native predators. Mar Biol 160:1529–1541
Hedgecock D (1994) Does variance in reproductive success limit effective population sizes of marine organisms. Genet Evol Aquat Organ 122:122–134
Hedgecock D, Pudovkin AI (2011) Sweepstakes reproductive success in highly fecund marine fish and shellfish: a review and commentary. Bull Mar Sci 87(4):971–1002
Hellberg ME (2009) Gene flow and isolation among populations of marine animals. Annu Rev Ecol Evol Syst 40:291–310
Hey J, Nielsen R (2004) Multilocus methods for estimating population sizes, migration rates and divergence time, with applications to the divergence of Drosophila pseudoobscura and D. persimilis. Genetics 167(2):747–760
Hoffman JI, Clarke A, Linse K, Peck LS (2011) Effects of brooding and broadcasting reproductive modes on the population genetic structure of two Antarctic gastropod molluscs. Mar Biol 158:287–296
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17(8):754–755
Ilves KL, Huang W, Wares JP, Hickerson MJ (2010) Colonization and/or mitochondrial selective sweeps across the North Atlantic intertidal assemblage revealed by multi-taxa approximate Bayesian computation. Mol Ecol 19:4505–4519
Jablonski D (1986) Larval ecology and macroevolution in marine invertebrates. Bull Mar Sci 39:565–587
Jablonski D, Lutz RA (1983) Larval ecology of marine benthic invertebrates: paleobiological implications. Biol Rev 58(1):21–89
Janson K (1987) Allozyme and shell variation in two marine snails (Littorina, Prosobranchia) with different dispersal abilities. Biol J Linn Soc Lond 30:245–256
Johannesson K (1988) The paradox of Rockall: why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar Biol 99:507–513
Johannesson K, Johannesson B (1995) Dispersal and population expansion in a direct developing marine snail (Littorina saxatilis) following a severe population bottleneck. Hydrobiologia 309:173–180
Johannesson K, Butlin RK, Panova M, Westram AM (2017) Mechanisms of adaptive divergence and speciation in Littorina saxatilis: integrating knowledge from ecology and genetics with new data emerging from genomic studies. In: Oleksiak MF, Rajora OP (eds) Population genomics: marine organisms. Springer International Publishing, Cham, pp 277–301
Johannesson K, Le Moan A, Perini S, André C (2020) A Darwinian laboratory of multiple contact zones. TREE 35:2730
Kess T, Galindo J, Boulding EG (2018) Genomic divergence between Spanish Littorina saxatilis ecotypes unravels limited admixture and extensive parallelism associated with population history. Ecol Evol 8:8311–8327
Kosheleva EA (2017) Genetic draft and linked selection in rapidly adapting populations. PhD diss., Harvard University, p 186
Kyle CJ, Boulding EG (2000) Comparative population genetic structure of marine gastropods (Littorina spp.) with and without pelagic larval dispersal. Mar Biol 137:835–845
Lee JEHY, Boulding EG (2009) Spatial and temporal population genetic structure of four northeastern Pacific littorinid gastropods: the effect of mode of larval development on variation at one mitochondrial and two nuclear DNA markers. Mol Ecol 18(10):2165–2184
Lourie SA, Green DM, Vincent AC (2005) Dispersal, habitat differences, and comparative phylogeography of Southeast Asian seahorses (Syngnathidae: Hippocampus). Mol Ecol 2005(14):1073–1094
Ludt WB, Rocha LA (2015) Shifting seas: the impacts of Pleistocene sea-level fluctuations on the evolution of tropical marine taxa. J Biogeogr 42(1):25–38
Luttikhuizen PC, Drent J, Baker AJ (2003) Disjunct distribution of highly diverged mitochondrial lineage clade and population subdivision in a marine bivalve with pelagic larval dispersal. Mol Ecol 12:2215–2229
Maggs CA, Castilho R, Foltz D, Henzler C, Jolly MT, Kelly J, Olsen J, Perez KE, Stam W, Väinölä R, Viard F, Wares J (2008) Evaluating signatures of glacial refugia for North Atlantic benthic marine taxa. Ecology 89:S108–S122
Marko PB (2004) ‘What’s larvae got to do with it?’ Disparate patterns of post-glacial population structure in two benthic marine gastropods with identical dispersal potential. Mol Ecol 13:597–611
Marko PB, Hart MW (2011) The complex analytical landscape of gene flow inference. Trends Ecol Evol 26(9):448–456
Marko PB, Hoffman JM, Emme SA, Mcgovern TM, Keever CC, Cox LN (2010) The ‘Expansion–Contraction’ model of Pleistocene biogeography: rocky shores suffer a sea change? Mol Ecol 19:146–169
Martel A, Chia FS (1991) Drifting and dispersal of small bivalves and gastropods with direct development. J Exp Mar Biol Ecol 26:131–147
Mertens LE, Treml EA, Von der Heyden S (2018) Genetic and biophysical models help define marine conservation focus areas. Front Mar Sci 6(5):268
Milá B, Van Tassell JL, Calderón JA, Rüber L, Zardoya R (2017) Cryptic lineage divergence in marine environments: genetic differentiation at multiple spatial and temporal scales in the widespread intertidal goby Gobiosoma bosc. Ecol Evol 7(14):5514–5523
Miller AW, Ruiz GM, Minton MS, Ambrose RF (2007) Differentiating successful and failed molluscan invaders in estuarine ecosystems. Mar Ecol Prog Ser 332:41–51
Moore PH (1977) Additions to the littoral fauna of Rockall, with a description of Araeolaimus penelope sp.nov. (Nematoda: Axonolaimidae). J Mar Biol Assoc UK 57:191–200
Morales HE, Faria R, Johanneson K, Larsson T, Panova M, Westram AM, Butlin RK (2019) Genomic architecture of parallel ecological divergence: beyond a single environmental contrast. Sci Adv 5:13
Ohara CC, Afflerbach JC, Scarborough C, Kaschner K, Halpern BS (2017) Aligning marine species range data to better serve science and conservation. PLoS ONE 12:e0175739
Panova M, Blakeslee AMH, Miller AW, Mäkinen T, Ruiz GM, Johannesson K, André C (2011) Glacial history of the north atlantic marine snail, Littorina saxatilis, inferred from distribution of mitochondrial DNA lineages. PLoS ONE 6:e17511
Pappalardo P, Fernández M (2014) Mode of larval development as a key factor to explain contrasting effects of temperature on species richness across oceans. Glob Ecol Biogeogr 23(1):12–23
Pechenik JA (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Prog Ser 11(177):269–297
Pelc RA, Warner RR, Gaines SD (2009) Geographical patterns of genetic structure in marine species with contrasting life histories. J Biogeogr 36:1881–1890
Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics 14:817–818
Reid DG (1996) Littorina littorea and Littorina saxatilis. In: Reid DG (ed) Systematics and evolution of Littorina. The Ray Society, London, pp 95–120
Reid DG, Rumbak E, Thomas RH (1996) DNA, morphology and fossils: phylogeny and evolutionary rates of the gastropod genus Littorina. Philos Trans R Soc B 351:877–895
Reid DG, Dyal P, Williams ST (2012) A global molecular phylogeny of 147 periwinkle species (Gastropoda, Littorininae). Zool Scr 41:125–136
Reitzel AM, Herrera S, Layden MJ, Martindale MQ, Shank TM (2013) Going where traditional markers have not gone before: utility of and promise of RAD sequencing in marine invertebrate phylogeography and population genomics. Mol Ecol 22:2953–2970
Riginos C, Douglas KE, Jin Y, Shanahan DF, Treml EA (2011) Effects of geography and life history traits on genetic differentiation in benthic marine fishes. Ecography 34(4):566–575
Ruiz GM, Fofonoff PW, Carlton JT, Wonham MJ, Hines AH (2000) Invasion of coastal marine communities in North America: apparent patterns, process, and biases. Annu Rev Ecol Syst 31:481–531
Scheltema RS (1986) On dispersal and planktonic larvae of benthic invertebrates: an eclectic overview and summary of problems. Bull Mar Sci 39:290–322
Selkoe KA, Toonen RJ (2011) Marine connectivity: a new look at pelagic larval duration and genetic metrics of dispersal. Mar Ecol Prog Ser 436:291–305
Shanks AL (2009) Pelagic larval duration and dispersal distance revisited. Biol Bull 216(3):373–385
Shen KN, Jamandre BW, Hsu CC, Tzeng WN, Durand JD (2011) Plio-Pleistocene sea level and temperature fluctuations in the northwestern Pacific promoted speciation in the globally-distributed flathead mullet Mugil cephalus. BMC Evol Biol 11(1):1–7
Steneck R, Carlton JT (2001) Human alterations of marine communities: students beware! In: Bertness M, Gaines S, Hay M (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 445–468
Tepolt CK, Blakeslee AM, Fowler AE, Darling JA, Torchin ME, Miller AW, Ruiz GM (2020) Strong genetic structure in a widespread estuarine crab: a test of potential versus realized dispersal. J Biogeogr 47:2532–2542
Teske PR, Papadopoulos I, Zardi GI, McQuaid CD, Edkins MT, Griffths CL, Barker NP (2007) Implications of life history for genetic structure and migration rates of southern African coastal invertebrates: planktonic, abbreviated and direct development. Mar Biol 152:697–711
Thiel M, Haye PA (2006) The ecology of rafting in the marine environment. III. Biogeographical and evolutionary consequences. Oceanogr Mar Biol Annu Rev 44:323–429
Wares JP, Cunningham CW (2001) Phylogeography and historical ecology of the North Atlantic intertidal. Evolution 55:2455–2469
Weber AT, Mérigot B, Valière S, Chenuil A (2015) Influence of the larval phase on connectivity: strong differences in the genetic structure of brooders and broadcasters in the Ophioderma longicauda species complex. Mol Ecol 24:6080–6094
Weersing K, Toonen RJ (2009) Population genetics, larval dispersal, and connectivity in marine systems. Mar Ecol Prog Ser 393:1–12
Acknowledgements
We thank the following for assistance and/or guidance during the data collection, analysis, and/or writing of the manuscript: I. Altman, G. Ashton, S. Brawley, C. Brown, J. Byers, J. Canning-Clode, J. Carlton, A. Fowler, C. Hamer, M. Lesser, SERC Invasions Lab. We also thank the Smithsonian Institution’s postdoctoral award and research funding provided to Blakeslee while at SERC, and we thank CeMEB (www.cemeb.science.gu.se), funded by the Swedish research councils VR and Formas, for financial support for Panova.
Funding
Research was supported by the Smithsonian Environmental Research Center (Blakeslee, Miller, Ruiz), East Carolina University (Blakeslee), and the University of Gothenberg (Johannesson, André, Panova).
Author information
Authors and Affiliations
Contributions
AB and MP: conceptualization, investigation, data curation, validation, visualization, formal analysis, draft preparation, draft review and draft editing. WM, GR, KJ, CA: conceptualization, validation, supervision, funding, draft review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors attest that there are no conflicts of interest. There was no use of live animals or human participants in this research.
Ethics approval
All data came from GENBANK and thus there was no use of live animals in this study.
Consent to participate and publish
All authors consent to participate and consent to publication.
Additional information
Responsible Editor: O. Puebla
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewers: A. Anh-Thu Weber and an undisclosed expert.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Blakeslee, A.M.H., Miller, A.W., Ruiz, G.M. et al. Population structure and phylogeography of two North Atlantic Littorina species with contrasting larval development. Mar Biol 168, 117 (2021). https://doi.org/10.1007/s00227-021-03918-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03918-8