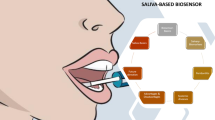
Abstract
The monitoring of stress levels in humans has become increasingly relevant, given the recent incline of stress-related mental health disorders, lifestyle impacts, and chronic physiological diseases. Long-term exposure to stress can induce anxiety and depression, heart disease, and risky behaviors, such as drug and alcohol abuse. Biomarker molecules can be quantified in biological fluids to study human stress. Cortisol, specifically, is a hormone biomarker produced in the adrenal glands with biofluid concentrations that directly correlate to stress levels in humans. The rapid, real-time detection of cortisol is necessary for stress management and predicting the onset of psychological and physical ailments. Current methods, including mass spectrometry and immunoassays, are effective for sensitive cortisol quantification. However, these techniques provide only single measurements which pose challenges in the continuous monitoring of stress levels. Additionally, these analytical methods often require trained personnel to operate expensive instrumentation. Alternatively, low-cost electrochemical biosensors enable the real-time detection and continuous monitoring of cortisol levels while also providing adequate analytical figures of merit (e.g., sensitivity, selectivity, sensor response times, detection limits, and reproducibility) in a simple design platform. This review discusses the recent developments in electrochemical biosensor design for the detection of cortisol in human biofluids. Special emphasis is given to biosensor recognition elements, including antibodies, molecularly imprinted polymers (MIPs), and aptamers, as critical components of electrochemical biosensors for cortisol detection. Furthermore, the advantages and limiting factors of various electrochemical techniques and sensing in complex biofluid matrices are overviewed. Remarks on the current challenges and future perspectives regarding electrochemical biosensors for stress monitoring are provided, including matrix effects (pH dependence and biological interferences), wearability, and large-scale production.
Graphical abstract







Similar content being viewed by others
References
Salleh MR. Life event, stress and illness. Malays J Med Sci. 2008;15:9–18.
Bethune S. More than a quarter of U.S. adults say they’re so stressed they can’t function. APA, American Psychological Association. 2022.
Iqbal T, Simpkin AJ, Roshan D, Glynn N, Killilea J, Walsh J, et al. Stress monitoring using wearable sensors: a pilot study and stress-predict dataset. Sensors (Basel). 2022;22:8135.
Kirsch DL, Woodbury-Farina MA. Stress in health and disease, an issue of psychiatric clinics of North America: Elsevier; 2014.
Zea M, Bellagambi FG, Ben Halima H, Zine N, Jaffrezic-Renault N, Villa R, et al. Electrochemical sensors for cortisol detections: almost there. TrAC Trends Anal Chem. 2020;132: 116058.
Sekar M, Sriramprabha R, Sekhar PK, Bhansali S, Ponpandian N, Pandiaraj M, et al. Review-towards wearable sensor platforms for the electrochemical detection of cortisol. J Electrochem Soc. 2020;167: 067508.
Rohleder N. Stress and inflammation – the need to address the gap in the transition between acute and chronic stress effects. Psychoneuroendocrinology. 2019;105:164–71.
Pruessner JC, Champagne F, Meaney MJ, Dagher A. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using [C-11]raclopride. J Neurosci. 2004;24:2825–31.
Jackson JA, Riordan HD, Neathery S, Revard C. Histamine levels in health and disease. J Orthomol Med. 1998;13:1947–60.
Hirsch D, Zukowska Z. NPY and stress 30 years later: the peripheral view. Cell Mol Neurobiol. 2012;32:645–59.
Taves MD, Gomez-Sanchez CE, Soma KK. Extra-adrenal glucocorticoids and mineralocorticoids: evidence for local synthesis, regulation, and function. Am J Physiol Endocrinol Metab. 2011;301:E11–24.
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2008;93:1526–40.
Kazakou P, Nicolaides NC, Chrousos GP. Basic concepts and hormonal regulators of the stress system. Horm Res Paediatr. 2023;96:8–16.
de Kloet ER, Joels M, Holsboer F. Stress and the brain: from adaptation to disease. Nat Rev Neurosci. 2005;6:463–75.
Levine A, Zagoory-Sharon O, Feldman R, Lewis JG, Weller A. Measuring cortisol in human psychobiological studies. Physiol Behav. 2007;90:43–53.
Mizoguchi K, Ishige A, Takeda S, Aburada M, Tabira T. Endogenous glucocorticoids are essential for maintaining prefrontal cortical cognitive function. J Neurosci. 2004;24:5492–9.
Papanicolaou DA, Mullen N, Kyrou I, Nieman LK. Nighttime salivary cortisol: a useful test for the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab. 2002;87:4515–21.
Corbalan-Tutau D, Madrid JA, Nicolas F, Garaulet M. Daily profile in two circadian markers “melatonin and cortisol” and associations with metabolic syndrome components. Physiol Behav. 2014;123:231–5.
Yehuda R, Teicher MH, Trestman RL, Levengood RA, Siever LJ. Cortisol regulation in posttraumatic stress disorder and major depression: a chronobiological analysis. Biol Psychiatry. 1996;40:79–88.
Adam EK, Vrshek-Schallhorn S, Kendall AD, Mineka S, Zinbarg RE, Craske MG. Prospective associations between the cortisol awakening response and first onsets of anxiety disorders over a six-year follow-up. Psychoneuroendocrinology. 2014;44:47–59.
Russell E, Koren G, Rieder M, Van Uum SH. The detection of cortisol in human sweat: implications for measurement of cortisol in hair. Ther Drug Monit. 2014;36:30–4.
Khumngern S, Jeerapan I. Advances in wearable electrochemical antibody-based sensors for cortisol sensing. Anal Bioanal Chem. 2023;415:3863–77.
Kraan GP, Dullaart RP, Pratt JJ, Wolthers BG, Drayer NM, De Bruin R. The daily cortisol production reinvestigated in healthy men. The serum and urinary cortisol production rates are not significantly different. J Clin Endocrinol Metab. 1998;83:1247–52.
Kerrigan JR, Veldhuis JD, Leyo SA, Iranmanesh A, Rogol AD. Estimation of daily cortisol production and clearance rates in normal pubertal males by deconvolution analysis. J Clin Endocrinol Metab. 1993;76:1505–10.
Esteban NV, Loughlin T, Yergey AL, Zawadzki JK, Booth JD, Winterer JC, et al. Daily cortisol production rate in man determined by stable isotope dilution/mass spectrometry. J Clin Endocrinol Metab. 1991;72:39–45.
Novak MA, Menard MT, El-Mallah SN, Rosenberg K, Lutz CK, Worlein J, et al. Assessing significant (>30%) alopecia as a possible biomarker for stress in captive rhesus monkeys (Macaca mulatta). Am J Primatol. 2017;79:1–8.
Incollingo Rodriguez AC, Epel ES, White ML, Standen EC, Seckl JR, Tomiyama AJ. Hypothalamic-pituitary-adrenal axis dysregulation and cortisol activity in obesity: a systematic review. Psychoneuroendocrinology. 2015;62:301–18.
Bjorntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–36.
Garcia-Blanco A, Diago V, Serrano De La Cruz V, Hervas D, Chafer-Pericas C, Vento M. Can stress biomarkers predict preterm birth in women with threatened preterm labor? Psychoneuroendocrinology. 2017;83:19–24.
Baid SK, Sinaii N, Wade M, Rubino D, Nieman LK. Radioimmunoassay and tandem mass spectrometry measurement of bedtime salivary cortisol levels: a comparison of assays to establish hypercortisolism. J Clin Endocrinol Metab. 2007;92:3102–7.
Casals G, Hanzu FA. Cortisol measurements in Cushing’s syndrome: immunoassay or mass spectrometry? Ann Lab Med. 2020;40:285–96.
Cao ZT, Wemm SE, Han L, Spink DC, Wulfert E. Noninvasive determination of human cortisol and dehydroepiandrosterone sulfate using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2019;411:1203–10.
Li C, Zhang Z, Liu X, Shen K, Gu P, Kang X. Simultaneous quantification of cortisol and cortisone in urines from infants with packed-fiber solid-phase extraction coupled to HPLC-MS/MS. J Chromatogr B: Analyt Technol Biomed Life Sci. 2017;1061:163–8.
Appel D, Schmid RD, Dragan CA, Bureik M, Urlacher VB. A fluorimetric assay for cortisol. Anal Bioanal Chem. 2005;383:182–6.
Posthuma-Trumpie GA, Korf J, van Amerongen A. Lateral flow (immuno)assay: its strengths, weaknesses, opportunities and threats. A literature survey. Anal Bioanal Chem. 2009;393:569–82.
Markossian S, Grossman A, Brimacombe K, Arkin M, Auld D, Austin C, et al. Immunoassay methods. The assay guidance manual. Bethesda (MD): Eli Lilly & Company and the National Center for Advancing Translational Sciences; 2004.
Haq N, Araque KA, Kanegusuku ALG, Wei B, Soldin SJ. Are serum cortisol measurements by immunoassays reliable?: A case series. Med Res Arch. 2020;8:2128.
Shimada M, Takahashi K, Ohkawa T, Segawa M, Higurashi M. Determination of salivary cortisol by ELISA and its application to the assessment of the circadian rhythm in children. Horm Res. 1995;44:213–7.
Stevens RC, Soelberg SD, Near S, Furlong CE. Detection of cortisol in saliva with a flow-filtered, portable surface plasmon resonance biosensor system. Anal Chem. 2008;80:6747–51.
Mejia-Salazar JR, Oliveira ON Jr. Plasmonic biosensing. Chem Rev. 2018;118:10617–25.
Choi J, Xue Y, Xia W, Ray TR, Reeder JT, Bandodkar AJ, et al. Soft, skin-mounted microfluidic systems for measuring secretory fluidic pressures generated at the surface of the skin by eccrine sweat glands. Lab Chip. 2017;17:2572–80.
Singh A, Kaushik A, Kumar R, Nair M, Bhansali S. Electrochemical sensing of cortisol: a recent update. Appl Biochem Biotechnol. 2014;174:1115–26.
Simoska O, Stevenson KJ. Electrochemical sensors for rapid diagnosis of pathogens in real time. Analyst. 2019;144:6461–78.
Renneberg R, Pfeiffer D, Lisdat F, Wilson G, Wollenberger U, Ligler F, et al. Frieder Scheller and the short history of biosensors. Adv Biochem Eng Biotechnol. 2008;109:1–18.
Simoska O, Stevenson KJ. Electrochemical sensors for detection of Pseudomonas aeruginosa virulence biomarkers: principles of design and characterization. Sens Actuators Rep. 2022;4:100072.
Karuppaiah G, Lee M-H, Bhansali S, Manickam P. Electrochemical sensors for cortisol detection: principles, designs, fabrication, and characterisation. Biosens Bioelectron 2023;239:115600.
Grieshaber D, MacKenzie R, Voros J, Reimhult E. Electrochemical biosensors - sensor principles and architectures. Sensors (Basel). 2008;8:1400–58.
Ye J-S, Ottova A, Tien HT, Sheu F-S. Nanostructured platinum-lipid bilayer composite as biosensor. Bioelectrochemistry. 2003;59:65–72.
Nikolelis DP, Siontorou CG. Hemoglobin modified bilayer lipid membranes (BLMs) biosensor for carbon dioxide detection Bioelectrochem Bioenerg. 1997;42:71–5.
Hazarika CS, D. Puzari, P. Medhi, T. Sharma, S. Use of cytochrome P450 enzyme isolated from Bacillus stratosphericus sp. as recognition element in designing schottky-based ISFET biosensor for hydrocarbon detection. IEEE Sens J 2018;18:6059–69.
Divya KP, Keerthana S, Viswanathan C, Ponpandian N. MXene supported biomimetic bilayer lipid membrane biosensor for zeptomole detection of BRCA1 gene. Microchim Acta. 2023;190:116.
Li Y, Si S, Huang F, Wei J, Dong S, Yang F, et al. Ultrasensitive label-free electrochemical biosensor for detecting linear microcystin-LR using degrading enzyme MlrB as recognition element. Bioelectrochemistry. 2022;144: 108000.
Coronado-Apodaca KG, Gonzalez-Meza GM, Aguayo-Acosta A, Araujo RG, Gonzalez-Gonzalez RB, Oyervides-Muñoz MA, et al. Immobilized enzyme-based novel biosensing system for recognition of toxic elements in the aqueous environment. Top Catal. 2023;66:606–24.
Lokar N, Pecar B, Mozek M, Vrtacnik D. Microfluidic electrochemical glucose biosensor with in situ enzyme immobilization. Biosensors (Basel). 2023;13:364.
Bhalla N, Jolly P, Formisano N, Estrela P. Introduction to biosensors. Essays Biochem. 2016;60:1–8.
Gillan L, Jansson E. Molecularly imprinted polymer on roll-to-roll printed electrodes as a single use sensor for monitoring of cortisol in sweat. Flex Print Electron. 2022;7: 025014.
Singh NK, Chung S, Sveiven M, Hall DA. Cortisol detection in undiluted human serum using a sensitive electrochemical structure-switching aptamer over an antifouling nanocomposite layer. ACS Omega. 2021;6:27888–97.
Rice P, Upasham S, Jagannath B, Manuel R, Pali M, Prasad S. CortiWatch: watch-based cortisol tracker. Future Sci OA. 2019;5:FSO416.
Sun K, Ramgir N, Bhansali S. An immunoelectrochemical sensor for salivary cortisol measurement. Sens Actuators B Chem. 2008;133:533–7.
Zubarev A, Cuzminschi M, Iordache AM, Iordache SM, Rizea C, Grigorescu CEA, et al. Graphene-based sensor for the detection of cortisol for stress level monitoring and diagnostics. Diagnostics (Basel). 2022;12:2593.
Olgac N, Karakus E, Yucel S, Liv L. Electrochemical biosensing of cortisol in a hormone tablet and artificial bodily fluids. Diam Relat Mater. 2023;132:109622.
Dhull N, Kaur G, Gupta V, Tomar M. Highly sensitive and non-invasive electrochemical immunosensor for salivary cortisol detection. Sens Actuators B Chem. 2019;293(281–288).
Simoska O, Minteer SD. Techniques in electroanalytical chemistry. ACS Publications: American Chemical Society; 2022.
Dhull N, Kaur G, Gupta V, Tomar M. Development of nanostructured nickel oxide thin film matrix by RF sputtering technique for the realization of efficient bioelectrode. Vacuum. 2018;158:68–74.
Liu J, Xu N, Men H, Li S, Lu Y, Low SS, Li X, Zhu L, Cheng C, Xu G, Liu Q. Salivary cortisol determination on smartphone-based differential pulse voltammetry system. Sensors. 2020;20:1422.
Cheng C, Li X, Xu G, Lu Y, Low SS, Liu G, et al. Battery-free, wireless, and flexible electrochemical patch for in situ analysis of sweat cortisol via near field communication. Biosens Bioelectron. 2021;172: 112782.
Yamaguchi M, Matsuda Y, Sasaki S, Sasaki M, Kadoma Y, Imai Y, et al. Immunosensor with fluid control mechanism for salivary cortisol analysis. Biosens Bioelectron. 2013;41:186–91.
Sanghavi BJ, Moore JA, Chavez JL, Hagen JA, Kelley-Loughnane N, Chou CF, et al. Aptamer-functionalized nanoparticles for surface immobilization-free electrochemical detection of cortisol in a microfluidic device. Biosens Bioelectron. 2016;78:244–52.
Nong C, Yang B, Li X, Feng S, Cui H. An ultrasensitive electrochemical immunosensor based on in-situ growth of CuWO4 nanoparticles on MoS2 and chitosan-gold nanoparticles for cortisol detection. Microchem J 2022;179:107434.
Lin KC, Jagannath B, Muthukumar S, Prasad S. Sub-picomolar label-free detection of thrombin using electrochemical impedance spectroscopy of aptamer-functionalized MoS2. Analyst. 2017;142:2770–80.
Khan MS, Dighe K, Wang Z, Srivastava I, Schwartz-Duval AS, Misra SK, et al. Electrochemical-digital immunosensor with enhanced sensitivity for detecting human salivary glucocorticoid hormone. Analyst. 2019;144:1448–57.
Daniels JS, Pourmand N. Label-free impedance biosensors: opportunities and challenges. Electroanalysis. 2007;19:1239–57.
Bertok T, Lorencova L, Chocholova E, Jane E, Vikartovska A, Kasak P, et al. Electrochemical impedance spectroscopy-based biosensors: mechanistic principles, analytical examples for assay of protein cancer biomarkers and challenges towards commercialization. ChemElectroChem. 2018;6:989–1003.
Sim D, Brothers MC, Slocik JM, Islam AE, Maruyama B, Grigsby CC, et al. Biomarkers and detection platforms for human health and performance monitoring: a review. Adv Sci (Weinh). 2022;9:2104426.
Liu W, Lian Z, Deng Q. Use of mean skin temperature in evaluation of individual thermal comfort for a person in a sleeping posture under steady thermal environment. Indoor Built Environ. 2015;24:489–99.
Benedek M, Kaernbach C. Physiological correlates and emotional specificity of human piloerection. Biol Psychol. 2011;86:320–9.
Kharitonov SA, Barnes PJ. Exhaled markers of pulmonary disease. Am J Respir Crit Care Med. 2001;163:1693–722.
Ryter SW, Choi AM. Carbon monoxide in exhaled breath testing and therapeutics. J Breath Res. 2013;7: 017111.
Dhama K, Latheef S, Daear M, Samad HA, Munjal A, Khandia R, et al. Biomarkers in stress related diseases/disorders: diagnostic, prognostic, and therapeutic values. Front Mol Biosci. 2019;6:91.
Pratico D, Rokach J, Lawson J, FitzGerald GA. F2-isoprostanes as indices of lipid peroxidation in inflammatory diseases. Chem Phys Lipids. 2004;128:165–71.
Venugopal M, Arya SK, Chornokur G, Bhansali S. A real-time and continuous assessment of cortisol in ISF using electrochemical impedance spectroscopy. Sens Actuators A Phys. 2011;172:154–60.
Pearlmutter P, DeRose G, Samson C, Linehan N, Cen Y, Begdache L, et al. Sweat and saliva cortisol response to stress and nutrition factors. Sci Rep. 2020;10:19050.
Sela-Culang I, Kunik V, Ofran Y. The structural basis of antibody-antigen recognition. Front Immunol. 2013;4:302.
Sharma S, Byrne H, O’Kennedy RJ. Antibodies and antibody-derived analytical biosensors. Essays Biochem. 2016;60:9–18.
Karachaliou CE, Koukouvinos G, Goustouridis D, Raptis I, Kakabakos S, Petrou P, et al. Cortisol immunosensors: a literature review. Biosensors (Basel). 2023;13:285.
Lee HB, Meeseepong M, Trung TQ, Kim BY, Lee NE. A wearable lab-on-a-patch platform with stretchable nanostructured biosensor for non-invasive immunodetection of biomarker in sweat. Biosens Bioelectron. 2020;156: 112133.
Torrente-Rodriguez RM, Tu J, Yang Y, Min J, Wang M, Song Y, et al. Investigation of cortisol dynamics in human sweat using a graphene-based wireless mHealth system. Matter. 2020;2:921–37.
Demuru S, Kim J, El Chazli M, Bruce S, Dupertuis M, Binz PA, et al. Antibody-coated wearable organic electrochemical transistors for cortisol detection in human sweat. ACS Sens. 2022;7:2721–31.
Madhu S, Ramasamy S, Manickam P, Nagamony P, Chinnuswamy V. TiO2 anchored carbon fibers as non-invasive electrochemical sensor platform for the cortisol detection Mater Lett. 2022;308:131238.
Gogotsi Y, Anasori B. The rise of MXenes. ACS Nano. 2019;18:8491–4.
Laochai T, Yukird J, Promphet N, Qin J, Chailapakul O, Rodthongkum N. Non-invasive electrochemical immunosensor for sweat cortisol based on L-cys/AuNPs/MXene modified thread electrode. Biosens Bioelectron. 2022;203: 114039.
Tian L, Jiang M, Su M, Cao X, Jiang Q, Liu Q, et al. Sweat cortisol determination utilizing MXene and multi-walled carbon nanotube nanocomposite functionalized immunosensor. Microchem J. 2023;185:108172.
Byrne B, Stack E, Gilmartin N, O’Kennedy R. Antibody-based sensors: principles, problems and potential for detection of pathogens and associated toxins. Sensors (Basel). 2009;9:4407–45.
Bonanno LM, Delouise LA. Steric crowding effects on target detection in an affinity biosensor. Langmuir. 2007;23:5817–23.
Tsekenis G, Chatzipetrou M, Massaouti M, Zergioti I. Comparative assessment of affinity based techniques for oriented antibody immobilization towards immunosensor performance optimization. J Sensors. 2019;2019:1–10.
Vasapollo G, Sole RD, Mergola L, Lazzoi MR, Scardino A, Scorrano S, et al. Molecularly imprinted polymers: present and future prospective. Int J Mol Sci. 2011;12:5908–45.
Yulianti ES, Rahman SF, Whulanza Y. Molecularly imprinted polymer-based sensor for electrochemical detection of cortisol. Biosensors (Basel). 2022;12:1090.
Parlak O, Keene ST, Marais A, Curto VF, Salleo A. Molecularly selective nanoporous membrane-based wearable organic electrochemical device for noninvasive cortisol sensing. Sci Adv. 2018;4:eaar2904.
Mugo SM, Alberkant J. Flexible molecularly imprinted electrochemical sensor for cortisol monitoring in sweat. Anal Bioanal Chem. 2020;412:1825–33.
Yeasmin S, Wu B, Liu Y, Ullah A, Cheng LJ. Nano gold-doped molecularly imprinted electrochemical sensor for rapid and ultrasensitive cortisol detection. Biosens Bioelectron. 2022;206: 114142.
Crapnell RD, Dempsey-Hibbert NC, Peeters M, Tridente A, Banks CE. Molecularly imprinted polymer based electrochemical biosensors: overcoming the challenges of detecting vital biomarkers and speeding up diagnosis. Talanta. 2020;2:100018.
Daniels E, Mustafa YL, Herdes C, Leese HS. Optimization of cortisol-selective molecularly imprinted polymers enabled by molecular dynamics simulations. ACS Appl Bio Mater. 2021;4:7243–53.
Zamora-Galvez A, Ait-Lahcen A, Mercante LA, Morales-Narvaez E, Amine A, Merkoci A. Molecularly imprinted polymer-decorated magnetite nanoparticles for selective sulfonamide detection. Anal Chem. 2016;88:3578–84.
Ansari S. Combination of molecularly imprinted polymers and carbon nanomaterials as a versatile biosensing tool in sample analysis: recent applications and challenges. TrAC Trends Anal Chem. 2017;93:134–51.
Nawaz N, Abu Bakar NK, Muhammad Ekramul Mahmud HN, Jamaludin NS. Molecularly imprinted polymers-based DNA biosensors. Anal Bioanal Chem. 2021;630:114328.
Ali GK, Omer KM. Molecular imprinted polymer combined with aptamer (MIP-aptamer) as a hybrid dual recognition element for bio(chemical) sensing applications. Review. Talanta. 2022;236: 122878.
Roushani M, Farokhi S, Rahmati Z. Development of a dual-recognition strategy for the aflatoxin B1 detection based on a hybrid of aptamer-MIP using a Cu2O NCs/GCE. Microchem J. 2022;178: 107328.
Pellitero MA, Shaver A, Arroyo-Curras N. Critical review—approaches for the electrochemical interrogation of DNA-based sensors: a critical review. J Electrochem Soc. 2020;167: 037529.
Liu Y, Canoura J, Alkhamis O, Xiao Y. Immobilization strategies for enhancing sensitivity of electrochemical aptamer-based sensors. ACS Appl Mater Interfaces. 2021;13:9491–9.
Jayasena SD. Aptamers: an emerging class of molecules that rival antibodies in diagnostics. Clin Chem. 1999;45:1628–50.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10.
Ganguly A, Lin KC, Muthukumar S, Prasad S. Autonomous, real-time monitoring electrochemical aptasensor for circadian tracking of cortisol hormone in sub-microliter volumes of passively eluted human sweat. ACS Sens. 2021;6:63–72.
Pusomjit P, Teengam P, Thepsuparungsikul N, Sanongkiet S, Chailapakul O. Impedimetric determination of cortisol using screen-printed electrode with aptamer-modified magnetic beads. Microchim Acta. 2021;188:41.
Mugo SM, Alberkant J, Bernstein N, Zenkina OV. Flexible electrochemical aptasensor for cortisol detection in human sweat. Anal Methods. 2021;13:4169–73.
Pali M, Jagannath B, Lin K-C, Upasham S, Sankhalab D, Upashama S. CATCH (cortisol Apta WATCH): ‘bio-mimic alarm’ to track anxiety, stress, immunity in human sweat. Electrochim Acta. 2021;390: 138834.
Wang B, Zhao C, Wang Z, Yang KA, Cheng X, Liu W, et al. Wearable aptamer-field-effect transistor sensing system for noninvasive cortisol monitoring. Sci Adv. 2022;8:1–15.
Sharma V, Sharma TK, Kaur I. Electrochemical detection of cortisol using a structure-switching aptamer immobilized on gold nanoparticles-modifed screen-printed electrodes. J Appl Electrochem. 2023;53:1765–76.
Singh NK, Chung S, Chang A-Y, Wang J, Hall DA. A non-invasive wearable stress patch for real-time cortisol monitoring using a pseudoknot-assisted aptamer. Biosens Bioelectron. 2023;227:115097.
Su T, Mi Z, Xia Y, Jin D, Xu Q, Hu X, et al. A wearable sweat electrochemical aptasensor based on the Ni-Co MOF nanosheet-decorated CNTs/PU film for monitoring of stress biomarker. Talanta. 2023;260: 124620.
Huang Z, Chen H, Ye H, Chen Z, Jaffrezic-Renault N, Guo Z. An ultrasensitive aptamer-antibody sandwich cortisol sensor for the noninvasive monitoring of stress state. Biosens Bioelectron. 2021;190: 113451.
Ran J, Luo D, Liu B. A dual-mode biosensor for salivary cortisol with antibody-aptamer sandwich pattern and enzyme catalytic amplification. J Solid State Electrochem. 2023;27:399–408.
Schoen I, Krammer H, Braun D. Hybridization kinetics is different inside cells. PNAS. 2009;106:21649–54.
Watkins Z, Karajic AY, Young T, White R, Heikenfeld J. Week-long operation of electrochemical aptamer sensors: new insights into self-assembled monolayer degradation mechanisms and solutions for stability in serum at body temperature. ACS Sens. 2023;8:1119–31.
Dunn MR, Jimenez RM, Chaput JC. Analysis of aptamer discovery and technology. Nat Rev Chem. 2017;1:0076.
Downs AM, Plaxco KW. Real-time, in vivo molecular monitoring using electrochemical aptamer based sensors: opportunities and challenges. ACS Sens. 2022;7:2823–32.
Shaver A, Curtis SD, Arroyo-Curras N. Alkanethiol monolayer end groups affect the long-term operational stability and signaling of electrochemical, aptamer-based sensors in biological fluids. ACS Appl Mater Interfaces. 2020;12:11214–23.
Santos-Cancel M, White RJ. Collagen membranes with ribonuclease inhibitors for long-term stability of electrochemical aptamer-based sensors employing RNA. Anal Chem. 2017;89:5598–604.
Clark V, Pellitero MA, Arroyo-Curras N. Explaining the decay of nucleic acid-based sensors under continuous voltammetric interrogation. Anal Chem. 2023;95:4974–83.
Pellitero MA, Nandini K, Sczepanski J, Arroyo-Curras N. Os(ii/iii) complex supports pH-insensitive electrochemical DNA-based sensing with superior operational stability than the benchmark methylene blue reporter. Analyst. 2023;148:806–13.
Li S, Ferrer-Ruiz A, Dai J, Ramos-Soriano J, Du X, Zhu M, et al. A pH-independent electrochemical aptamer-based biosensor supports quantitative, real-time measurement in vivo. Chem Sci. 2022;13:8813–20.
Arroyo-Currás N, Dauphin-Ducharme P, Scida K, Chávez JL. From the beaker to the body: translational challenges for electrochemical, aptamer-based sensors. Anal Methods. 2020;12:1288–310.
Ricci F, Zari N, Caprio F, Recine S, Amine A, Moscone D, et al. Surface chemistry effects on the performance of an electrochemical DNA sensor. Bioelectrochemistry. 2009;76:208–13.
Castiglione V, Aimo A, Vergaro G, Saccaro L, Passino C, Emdin M. Biomarkers for the diagnosis and management of heart failure. Heart Fail Rev. 2022;27:625–43.
Shajari S, Salahandish R, Zare A, Hassani M, Moossavi S, Munro E, et al. MicroSweat: a wearable microfluidic patch for noninvasive and reliable sweat collection enables human stress monitoring. Adv Sci (Weinh). 2023;10:2204171.
Mirica KA. Materials matter: adsvancing sensor science through innovation in materials chemistry. ACS Sens. 2022;7:3580–1.
Acknowledgements
The authors gratefully acknowledge financial support from the University of South Carolina.
Funding
This work was supported by start-up funding (to O.S.) from the University of South Carolina.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Source of biological material
Not applicable.
Statement on animal welfare
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Weber, C.J., Clay, O.M., Lycan, R.E. et al. Advances in electrochemical biosensor design for the detection of the stress biomarker cortisol. Anal Bioanal Chem 416, 87–106 (2024). https://doi.org/10.1007/s00216-023-05047-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-023-05047-1