Abstract
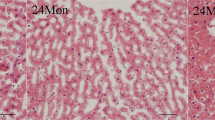
Aging is a physiological process in which there is a progressive decline of function in multiple organs such as the liver. The development of natural therapies, such as sericin, for delaying age-associated diseases is of major interest in this regard. Twenty-seven mice were divided into three groups of nine, including young control group (8 weeks, received normal saline), aged control group (24 months, received normal saline), and sericin-treated aged mice (24 months, received sericin at dose 100 mg/kg/day) via oral administration for 14 days. The liver enzymes in serum and oxidative stress markers in liver tissue were evaluated using spectrophotometric/ELISA methods. Apoptotic proteins, pro-inflammatory cytokines, COX2, JNK, and P-38 levels were assessed by western blot analysis. β-galactosidase expression was determined by a qRT-PCR method. The findings showed that 100 mg/kg of sericin reduced liver enzymes in aged mice. Antioxidant capacity in treated aged mice showed an improvement in all indexes in the liver tissue. Also, sericin administration declined pro-inflammatory markers to varying degrees in aged-treated mice. Sericin also increased the expression level of Bcl-2 and decreased the expression level of Bax and cleaved caspase-3.In addition, treatment with sericin suppressed protein expression of p-JNK and p-JNK/JNK. Collectively, these findings would infer that sericin administration may have a hepatoprotective effect in aging-induced liver damage in mice.







Similar content being viewed by others
Availability of data and materials
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Fathi E, Farahzadi R (2016) Isolation, culturing, characterization and aging of adipose tissue-derived mesenchymal stem cells: a brief overview. Braz Arch Biol Technol 59
López-Otín C et al (2013) The hallmarks of aging. Cell 153(6):1194–1217
Fathi E, et al (2019) Telomere shortening as a hallmark of stem cell senescence. Stem Cell Investig 6
Ebrahimi V et al (2020) Epigenetic modifications in gastric cancer: focus on DNA methylation. Gene. 742:144577
Farahzadi R et al (2016) L-carnitine effectively induces hTERT gene expression of human adipose tissue-derived mesenchymal stem cells obtained from the aged subjects. Int J Stem Cells 9(1):107–114
Kirkwood TB (2005) Understanding the odd science of aging. Cell 120(4):437–447
Farahzadi R, Fathi E, Vietor I (2020) Mesenchymal stem cells could be considered as a candidate for further studies in cell-based therapy of Alzheimer’s disease via targeting the signaling pathways. ACS Chem Neurosci 11(10):1424–1435
Fathi E, Sanaat Z, Farahzadi R (2019) Mesenchymal stem cells in acute myeloid leukemia: a focus on mechanisms involved and therapeutic concepts. Blood Research 54(3):165–174
Kaeberlein M (2013) Longevity and aging. F1000prime Rep 5
Mobarak H et al (2017) L-carnitine significantly decreased aging of rat adipose tissue-derived mesenchymal stem cells. Vet Res Commun 41(1):41–47
Mehdizadeh A et al (2017) Liposome-mediated RNA interference delivery against Erk1 and Erk2 does not equally promote chemosensitivity in human hepatocellular carcinoma cell line HepG2. Artif Cells, Nanomed Biotechnol 45(8):1612–1619
Rui L (2011) Energy metabolism in the liver. Compr Physiol 4(1):177–197
Kim H, Kisseleva T, Brenner DA (2015) Aging and liver disease. Curr Opin Gastroenterol 31(3):184
Basso A et al (1998) Reduced DNA synthesis in primary cultures of hepatocytes from old mice is restored by thymus grafts. J Gerontol A Biol Sci Med Sci 53(2):B111–B116
Wu I-C et al (2014) Oxidative stress and frailty: a closer look at the origin of a human aging phenotype. Aging. Elsevier, pp 3–14
Jang JY et al (2018) The role of mitochondria in aging. J Clin Investig 128(9):3662–3670
Jones DP (2008) Radical-free biology of oxidative stress. Am J Physiol Cell Physiol 295(4):C849–C868
Kroemer G, Galluzzi L, Brenner C (2007) Mitochondrial membrane permeabilization in cell death. Physiol Rev 87(1):99–163
Green DR, Galluzzi L, Kroemer G (2011) Mitochondria and the autophagy–inflammation–cell death axis in organismal aging. Science 333(6046):1109–1112
Rezabakhsh A et al (2017) Effect of hydroxychloroquine on oxidative/nitrosative status and angiogenesis in endothelial cells under high glucose condition. BioImpacts. 7(4):219
Feizy N et al (2016) Morphine inhibited the rat neural stem cell proliferation rate by increasing neuro steroid genesis. Neurochem Res 41(6):1410–1419
Saliani N, Montazersaheb S, Kouhsari SM (2017) Micromanaging glucose tolerance and diabetes. Adv Pharm Bullet 7(4):547
Bejma J, Ramires P, Ji L (2000) Free radical generation and oxidative stress with ageing and exercise: differential effects in the myocardium and liver. Acta Physiol Scand 169(4):343–351
Szabo G, Csak T (2012) Inflammasomes in liver diseases. J Hepatol 57(3):642–654
Lasry A, Ben-Neriah Y (2015) Senescence-associated inflammatory responses: aging and cancer perspectives. Trends Immunol 36(4):217–228
Di Iorio A et al (2003) Serum IL-1β levels in health and disease: a population-based study. ‘The InCHIANTI study.’ Cytokine. 22(6):198–205
Wunderlich FT et al (2010) Interleukin-6 signaling in liver-parenchymal cells suppresses hepatic inflammation and improves systemic insulin action. Cell Metab 12(3):237–249
Williams LM et al (2008) Rac mediates TNF-induced cytokine production via modulation of NF-κB. Mol Immunol 45(9):2446–2454
Kyriakis JM, Avruch J (2001) Mammalian mitogen-activated protein kinase signal transduction pathways activated by stress and inflammation. Physiol Rev 81(2):807–869
Roux PP, Blenis J (2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 68(2):320–344
Schwabe RF, Brenner DA (2006) Mechanisms of liver injury. I. TNF-α-induced liver injury: role of IKK, JNK, and ROS pathways. Am J Physiol-Gastrointest Liver Physiol. 290(4):G583–G589
Rajawat YS, Hilioti Z, Bossis I (2009) Aging: central role for autophagy and the lysosomal degradative system. Ageing Res Rev 8(3):199–213
Carmona-Gutierrez D et al (2016) The crucial impact of lysosomes in aging and longevity. Ageing Res Rev 32:2–12
Morgunova G et al (2015) Senescence-associated β-galactosidase—a biomarker of aging, DNA damage, or cell proliferation restriction? Mosc Univ Biol Sci Bull 70(4):165–167
Ding A-J et al (2017) Current perspective in the discovery of anti-aging agents from natural products. Nat Prod Bioprospect 7(5):335–404
Deori M et al (2016) Antioxidant effect of sericin in brain and peripheral tissues of oxidative stress induced hypercholesterolemic rats. Front Pharmacol 7:319
Seyedaghamiri F, et al (2021) Sericin modulates learning and memory behaviors by tuning of antioxidant, inflammatory, and apoptotic markers in the hippocampus of aged mice. Mol Biol Rep 1–12
Kunz RI, et al (2016) Silkworm sericin: properties and biomedical applications. BioMed Res Int 2016
Barajas-Gamboa JA et al (2016) Sericin applications: a globular silk protein. Ingeniería y Competitividad 18(2):193–206
Bagheri Y, et al (2020) Protective effects of gamma oryzanol on distant organs after kidney ischemia-reperfusion in rats: a focus on liver protection. Human Exp Toxicol 0960327120979014
Bagheri Y et al (2021) Comparative study of gavage and intraperitoneal administration of gamma-oryzanol in alleviation/attenuation in a rat animal model of renal ischemia/reperfusion-induced injury. Iran J Basic Med Sci 24(2):175–183
Rahimi M, et al (2021) Renoprotective effects of prazosin on ischemia-reperfusion injury in rats. Human Exp Toxicol. 0960327121993224
Montazersaheb S et al (2019) Downregulation of TdT expression through splicing modulation by antisense peptide nucleic acid (PNA). Curr Pharm Biotechnol 20(2):168–178
Tarhriz V et al (2019) Transient induction of Cdk9 in the early stage of differentiation is critical for myogenesis. J Cell Biochem 120(11):18854–18861
Fathi E, Farahzadi R, Valipour B (2021) Alginate/gelatin encapsulation promotes NK cells differentiation potential of bone marrow resident C-kit(+) hematopoietic stem cells. Int J Biol Macromol 177:317–327
Tarhriz V et al (2018) CDK9 regulates apoptosis of myoblast cells by modulation of microRNA-1 expression. J Cell Biochem 119(1):547–554
Fathi E, et al (2020) L-carnitine extends the telomere length of the cardiac differentiated CD117+-expressing stem cells. Tissue and Cell 101429
Fathi E et al (2020) Cardiac differentiation of bone-marrow-resident c-kit+ stem cells by L-carnitine increases through secretion of VEGF, IL6, IGF-1, and TGF-β as clinical agents in cardiac regeneration. J Biosci 45(1):1–11
Kato N et al (1998) Silk protein, sericin, inhibits lipid peroxidation and tyrosinase activity. Biosci Biotechnol Biochem 62(1):145–147
Prasong S (2011) Screening of antioxidant activity of some Samia ricini (Eri) silks: comparison with Bombyx mori. J Biol Sci 11(4):336–339
Yagi K (1998) Simple assay for the level of total lipid peroxides in serum or plasma. Free radical and antioxidant protocols. Springer, pp 101–106
Armstrong D, Browne R (1994) The analysis of free radicals, lipid peroxides, antioxidant enzymes and compounds related to oxidative stress as applied to the clinical chemistry laboratory. Free radicals in diagnostic medicine. Springer, pp 43–58
Walter MF et al (2004) Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: a longitudinal analysis of the PREVENT study. J Am Coll Cardiol 44(10):1996–2002
Chlapanidas T et al (2013) Sericins exhibit ROS-scavenging, anti-tyrosinase, anti-elastase, and in vitro immunomodulatory activities. Int J Biol Macromol 58:47–56
Aramwit P et al (2009) Monitoring of inflammatory mediators induced by silk sericin. J Biosci Bioeng 107(5):556–561
Dash R et al (2008) Silk sericin protein of tropical tasar silkworm inhibits UVB-induced apoptosis in human skin keratinocytes. Mol Cell Biochem 311(1–2):111–119
Pearson G et al (2001) Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev 22(2):153–183
Sadler KC et al (2004) MAP kinases regulate unfertilized egg apoptosis and fertilization suppresses death via Ca2+ signaling. Mol Reprod Dev 67(3):366–383
Tarín JJ, Pérez-Albalá S, Cano A (2001) Cellular and morphological traits of oocytes retrieved from aging mice after exogenous ovarian stimulation. Biol Reprod 65(1):141–150
Qin H, et al (2020) Safety assessment of water-extract sericin from silkworm (Bombyx mori) cocoons using different model approaches. 2020
Kunz RI, et al (2016) Silkworm sericin: properties and biomedical applications. 2016
SASAKI M, et al (2000) A resistant protein, sericin improves atropine-induced constipation in rats. 6(4): 280–283
Funding
The financial sponsorship of this work was provided by the Molecular Medicine Research Center, Tabriz University of Medical Sciences, Tabriz, Iran (Pazhoohan ID., 65705; ethical code No., IR.TBZMED.VCR.REC.1399.334).
Author information
Authors and Affiliations
Contributions
BY and S-SE conceptualized and designed the experiment. BY and MJ conducted experiments. BY, AA, JN-N, MZ, and MK contributed new reagents and analyzed data. MS, S-SE, and FE supervised the project. MS, S-SE, and FE wrote, reviewed, and edited the manuscript. All listed authors have read and approved the final manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Ethics approval
This ethical code of this project is IR.TBZMED.VCR.REC.1399.334.
Consent to participate
This is an animal study.
Consent for publication
All authors agree to publish.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bagheri, Y., Sadigh-Eteghad, S., Fathi, E. et al. Hepatoprotective effects of sericin on aging-induced liver damage in mice. Naunyn-Schmiedeberg's Arch Pharmacol 394, 2441–2450 (2021). https://doi.org/10.1007/s00210-021-02160-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-021-02160-9