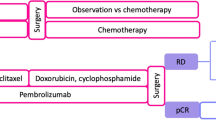
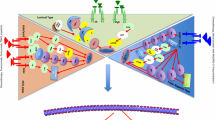
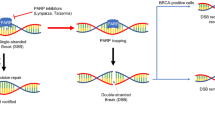
Abstract
Breast cancer (BC) is predominantly diagnosed and is a major cause of malignancy-related death among women globally. Traditional BC therapies include surgical excision of damaged tissue, radiation therapy, systemic chemotherapy, and endocrine therapy. BC typically targets many important pathways, including the hormonal receptors, HER2, and PI3K/AKT/mTOR signaling pathways. Other therapy strategies include blocking immunological checkpoints, addressing DNA repair, and developing antibody–drug conjugates. As technology advances, the approach to BC therapy has changed from using toxic, high dosages of drugs to using some novel targeted therapies like biomarker-specific, line-agnostic, and receptor-agnostic. The success of Trastuzumab (Herceptin) in BC therapy has prepared the path for a next-generation strategy to target the tumor's molecular mechanism. Research is in progress to identify novel blood-based and non-blood-based biomarkers for early detection and therapy for BC. Tumor heterogeneity, on the other hand, remains a major challenge. Drug repositioning and advanced formulation strategies are also on the list of next-generation therapies for BC. It is necessary to thoroughly analyze the patient’s mindset, financial capacity, and utility of innovative medical attention to effectively develop next-generation cancer medications. This chapter focuses on next-generation therapeutic strategies for the treatment of BC.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Abd-Elnaby M, Alfonse M, Roushdy M (2021) Classification of breast cancer using microarray gene expression data: a survey. J Biomed Inform 117:103764. https://doi.org/10.1016/j.jbi.2021.103764
Abdel-Fatah T, Broom RJ, Lu J, Moseley PM, Huang B, Li L, Liu S, Chen L, Ma RZ, Cao W (2019) SHON expression predicts response and relapse risk of breast cancer patients after anthracycline-based combination chemotherapy or tamoxifen treatment. Br J Cancer 120(7):728–745. https://doi.org/10.1038/s41416-019-0405-x
Abrahimi MS, Elwood M, Lawrenson R, Campbell I, Tin Tin S (2021) Associated factors and survival outcomes for breast conserving surgery versus mastectomy among New Zealand women with early-stage breast cancer. Int J Environ Health Res 18(5):2738. https://doi.org/10.3390/ijerph18052738
Aggarwal V, Priyanka K, Tuli HS (2020) Emergence of circulating MicroRNAs in breast cancer as diagnostic and therapeutic efficacy biomarkers. Mol Diagn Ther 24(2):153–173. https://doi.org/10.1007/s40291-020-00447-w
Ahmad A (2019) Breast cancer statistics: recent trends. Breast Cancer Metastasis Drug Resist:1–7. https://doi.org/10.1007/978-3-030-20301-6_1
Ambrosone CB, Young AC, Sucheston LE, Wang D, Li Y, Liu S, Tang L, Hu Q, Freudenheim JL, Shields PG (2014) Genome-wide methylation patterns provide insight into differences in breast tumor biology between American women of African and European ancestry. Oncotarget 5(1):237. https://doi.org/10.18632/oncotarget.1599
Andreopoulou E, Schweber SJ, Sparano JA, McDaid HM (2015) Therapies for triple negative breast cancer. Expert Opin Pharmacother 16(7):983–998. https://doi.org/10.1517/14656566.2015.1032246
Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, Boccardo F, Boddington C, Bogaerts J, Bonadonna G (2018) Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol 19(1):27–39. https://doi.org/10.1016/S1470-2045(17)30777-5
Bal A, Joshi K (2022) Pathology of breast cancer. Breast Cancer, Springer:125–138. https://doi.org/10.1007/978-981-16-4546-4_8
Bayraktar S, Glück S (2013) Molecularly targeted therapies for metastatic triple-negative breast cancer. Breast Cancer Res Treat 138(1):21–35. https://doi.org/10.1007/s10549-013-2421-5
Beretov J, Wasinger VC, Graham PH, Millar EK, Kearsley JH, Li Y (2014) Proteomics for breast cancer urine biomarkers. Adv Clin Chem 63:123–167. https://doi.org/10.1016/B978-0-12-800094-6.00004-2
Blum JL, Flynn PJ, Yothers G, Asmar L, Geyer CE Jr, Jacobs SA, Robert NJ, Hopkins JO, O’Shaughnessy JA, Dang CT (2017) Anthracyclines in early breast cancer: The abc trials—usor 06–090, nsabp b-46-i/usor 07132, and nsabp b-49 (nrg oncology). J Clin Oncol 35(23):2647. https://doi.org/10.1200/JCO.2016.71.4147
Buqué A, Bloy N, Perez-Lanzón M, Iribarren K, Humeau J, Pol JG, Levesque S, Mondragon L, Yamazaki T, Sato A (2020) Immunoprophylactic and immunotherapeutic control of hormone receptor-positive breast cancer. Nat Commun 11(1):1–18. https://doi.org/10.1038/s41467-020-17644-0
Buszewski B, Kęsy M, Ligor T, Amann A (2007) Human exhaled air analytics: biomarkers of diseases. Biomed Chromatogr 21(6):553–566. https://doi.org/10.1002/bmc.835
Cejalvo JM, Pascual T, Fernández-Martínez A, Brasó-Maristany F, Gomis RR, Perou CM, Muñoz M, Prat A (2018) Clinical implications of the non-luminal intrinsic subtypes in hormone receptor-positive breast cancer. Cancer Treat Rev 67:63–70. https://doi.org/10.1016/j.ctrv.2018.04.015
Chan AA, Bashir M, Rivas MN, Duvall K, Sieling PA, Pieber TR, Vaishampayan PA, Love SM, Lee DJ (2016) Characterization of the microbiome of nipple aspirate fluid of breast cancer survivors. Sci Rep 6(1):1–11. https://doi.org/10.1038/srep28061
Chartron E, Theillet C, Guiu S, Jacot W (2019) Targeting homologous repair deficiency in breast and ovarian cancers: biological pathways, preclinical and clinical data. Crit Rev Oncol Hematol 133:58–73. https://doi.org/10.1016/j.critrevonc.2018.10.012
Costa RL, Czerniecki BJ (2020) Clinical development of immunotherapies for HER2+ breast cancer: a review of HER2-directed monoclonal antibodies and beyond. NPJ Breast Cancer 6(1):1–11. https://doi.org/10.1038/s41523-020-0153-3
Dayon L, Cominetti O, Affolter M (2022) Proteomics of human biological fluids for biomarker discoveries: technical advances and recent applications. Expert Rev Proteom 19(2):131–151. https://doi.org/10.1080/14789450.2022.2070477
De A, Kuppusamy G (2020) Metformin in breast cancer: preclinical and clinical evidence. Curr Probl Cancer 44(1):100488. https://doi.org/10.1016/j.currproblcancer.2019.06.003
De A, Kuppusamy G, Karri VVSR (2018) Affibody molecules for molecular imaging and targeted drug delivery in the management of breast cancer. Int J Biol Macromol 107:906–919. https://doi.org/10.1016/j.ijbiomac.2017.09.059
Ding Q, Huo L, Peng Y, Yoon EC, Li Z, Sahin AA (2022) Immunohistochemical markers for distinguishing metastatic breast carcinoma from other common malignancies: update and revisit. Semin Diagn Pathol Elsevier. https://doi.org/10.1053/j.semdp.2022.04.002
Dong MP, Enomoto M, Thuy LTT, Hai H, Hieu VN, Hoang DV, Iida-Ueno A, Odagiri N, Amano-Teranishi Y, Hagihara A (2020) Clinical significance of circulating soluble immune checkpoint proteins in sorafenib-treated patients with advanced hepatocellular carcinoma. Sci Rep 10(1):1–10. https://doi.org/10.1038/s41598-020-60440-5
Duffy MJ (2006) Serum tumor markers in breast cancer: are they of clinical value? Clin Chem 52(3):345–351. https://doi.org/10.1373/clinchem.2005.059832
Fakhar HBZ, Rezaei-Tavirani M, Akbari ME, Sajadi SN, HadiZadeh M. Proteins which have been found in breast cancer by proteomic’s analyzer. https://doi.org/10.46718/JBGSR.2022.11.000271
Feng Y, Spezia M, Huang S, Yuan C, Zeng Z, Zhang L, Ji X, Liu W, Huang B, Luo W (2018) Breast cancer development and progression: Risk factors, cancer stem cells, signaling pathways, genomics, and molecular pathogenesis. Genes Dis 5(2):77–106. https://doi.org/10.1016/j.gendis.2018.05.001
Fraguas-Sánchez A, Martín-Sabroso C, Fernández-Carballido A, Torres-Suárez A (2019) Current status of nanomedicine in the chemotherapy of breast cancer. Cancer Chemother Pharmacol 84(4):689–706. https://doi.org/10.1007/s00280-019-03910-6
Garreffa E, Arora D (2021) Breast cancer in the elderly, in men and during pregnancy. Surg Infect (Larchmt):139–146. https://doi.org/10.1016/j.mpsur.2021.11.018
George JN, Nester CM (2014) Syndromes of thrombotic microangiopathy. N Engl J Med 371(7):654–666. https://doi.org/10.1056/NEJMra1312353
Gombos A, Franzoi MA, Awada A (2019) Investigational drugs in early stage clinical trials for the treatment of HER2+ breast cancer. Expert Opin Investig Drugs 28(7):617–627. https://doi.org/10.1080/13543784.2019.1633306
Guo G, Zhang F, Gao R, Delsite R, Feng Z, Powell SN (2011) DNA repair and synthetic lethality. Int J Oral Sci 3(4):176–179. https://doi.org/10.4248/IJOS11064
Guo Q, Lin X, Ye L, Xu R, Dai Y, Zhang Y, Chen Q (2019) Comparative efficacy of CDK4/6 inhibitors plus aromatase inhibitors versus fulvestrant for the first-line treatment of hormone receptor-positive advanced breast cancer: a network meta-analysis. Target Oncol 14(2):139–148. https://doi.org/10.1007/s11523-019-00633-9
Hamam R, Hamam D, Alsaleh KA, Kassem M, Zaher W, Alfayez M, Aldahmash A, Alajez NM (2017) Circulating microRNAs in breast cancer: novel diagnostic and prognostic biomarkers. Cell Death Dis 8(9):e3045–e3045. https://doi.org/10.1038/cddis.2017.440
Heichman KA, Warren JD (2012) DNA methylation biomarkers and their utility for solid cancer diagnostics. Clin Chem Lab Med 50(10):1707–1721. https://doi.org/10.1515/cclm-2011-0935
Hodgson MC, Shao L-J, Frolov A, Li R, Peterson LE, Ayala G, Ittmann MM, Weigel NL, Agoulnik IU (2011) Decreased expression and androgen regulation of the tumor suppressor gene INPP4B in prostate cancer androgen regulation of INPP4B. Cancer Res 71(2):572–582. https://doi.org/10.1158/0008-5472.CAN-10-2314
Horvath E (2021) Molecular subtypes of breast cancer-What breast imaging radiologists need to know. Rev Chil Radiol 27(1):17–26
Houghton SC, Hankinson SE (2021) Cancer progress and priorities: breast cancer. Cancer Epidemiol Biomarkers Prev 30(5):822–844. https://doi.org/10.1158/1055-9965.EPI-20-1193
Hurvitz SA, Hu Y, O’Brien N, Finn RS (2013) Current approaches and future directions in the treatment of HER2-positive breast cancer. Cancer Treat Rev 39(3):219–229. https://doi.org/10.1016/j.ctrv.2012.04.008
Huveldt D, Lewis-Tuffin LJ, Carlson BL, Schroeder MA, Rodriguez F, Giannini C, Galanis E, Sarkaria JN, Anastasiadis PZ (2013) Targeting Src family kinases inhibits bevacizumab-induced glioma cell invasion. PLoS ONE 8(2):e56505. https://doi.org/10.1371/journal.pone.0056505
Ilic L, Haidinger G, Simon J, Hackl M, Schernhammer E, Papantoniou K (2022) Trends in female breast cancer incidence, mortality, and survival in Austria, with focus on age, stage, and birth cohorts (1983–2017). Sci Rep 12(1):1–10. https://doi.org/10.1038/s41598-022-10560-x
Janeva S, Zhang C, Kovács A, Parris TZ, Crozier JA, Pezzi CM, Linderholm B, Audisio RA, Bagge RO (2020) Adjuvant chemotherapy and survival in women aged 70 years and older with triple-negative breast cancer: a Swedish population-based propensity score-matched analysis. Lancet Healthy Longevity 1(3):e117–e124. https://doi.org/10.1016/S2666-7568(20)30018-0
Jang MH, Kim HJ, Kim EJ, Chung YR, Park SY (2015) Expression of epithelial-mesenchymal transition–related markers in triple-negative breast cancer: ZEB1 as a potential biomarker for poor clinical outcome. Hum Pathol 46(9):1267–1274. https://doi.org/10.1016/j.humpath.2015.05.010
Jeyalatha MV, Qu Y, Liu Z, Ou S, He X, Bu J, Li S, Reinach PS, Liu Z, Li W (2017) Function of meibomian gland: contribution of proteins. Exp Eye Res 163:29–36. https://doi.org/10.1016/j.exer.2017.06.009
Joshi H, Press MF (2018) Molecular oncology of breast cancer. The Breast, Elsevier 282–307:e285. https://doi.org/10.1016/B978-0-323-35955-9.00022-2
Kasimir-Bauer S, Bittner A-K, König L, Reiter K, Keller T, Kimmig R, Hoffmann O (2016) Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res 18(1):1–15. https://doi.org/10.1186/s13058-016-0679-3
Kiepas A (2021) Defining the molecular mechanisms that regulate breast cancer cell migration, McGill University (Canada). https://www.proquest.com/dissertations-theses/defining-molecular-mechanisms-that-regulate/docview/2571092678/se-2
Korde LA, Somerfield MR, Carey LA, Crews JR, Denduluri N, Hwang ES, Khan SA, Loibl S, Morris EA, Perez A (2021) Neoadjuvant chemotherapy, endocrine therapy, and targeted therapy for breast cancer: ASCO guideline. J Clin Oncol 39(13):1485–1505. https://doi.org/10.1200/JCO.20.03399
Krop I, Ismaila N, Andre F, Bast RC, Barlow W, Collyar DE, Hammond ME, Kuderer NM, Liu MC, Mennel RG (2017) Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 35(24):2838. https://doi.org/10.1200/JCO.2017.74.0472
Lambert JM, Chari RV (2014) Ado-trastuzumab Emtansine (T-DM1): an antibody–drug conjugate (ADC) for HER2-positive breast cancer. ACS Publications 57(16):6949–6964. https://doi.org/10.1021/jm500766w
Lebrecht A, Boehm D, Schmidt M, Koelbl H, Schwirz RL, Grus FH (2009) Diagnosis of breast cancer by tear proteomic pattern. Cancer Genomics Proteomics 6(3):177–182
Li J, Guan X, Fan Z, Ching L-M, Li Y, Wang X, Cao W-M, Liu D-X (2020) Non-invasive biomarkers for early detection of breast cancer. Cancers 12(10):2767. https://doi.org/10.3390/cancers12102767
Li J, Peng Y, Duan Y (2013) Diagnosis of breast cancer based on breath analysis: an emerging method. Crit Rev Oncol Hematol 87(1):28–40. https://doi.org/10.1016/j.critrevonc.2012.11.007
Li X, Dai D, Chen B, Tang H, Xie X, Wei W (2018) Clinicopathological and prognostic significance of cancer antigen 15-3 and carcinoembryonic antigen in breast cancer: a meta-analysis including 12,993 patients. Disease Markers. https://doi.org/10.1155/2018/9863092
Liedtke C, Cardone L, Tordai A, Yan K, Gomez HL, Figureoa LJB, Hubbard RE, Valero V, Souchon EA, Symmans WF (2008) PIK3CA-activating mutations and chemotherapy sensitivity in stage II–III breast cancer. Breast Cancer Res 10(2):1–10. https://doi.org/10.1186/bcr1984
Linggi B, Carpenter G (2006) ErbB receptors: new insights on mechanisms and biology. Trends Cell Biol 16(12):649–656. https://doi.org/10.1016/j.tcb.2006.10.008
Lisec J, Schauer N, Kopka J, Willmitzer L, Fernie AR (2006) Gas chromatography mass spectrometry–based metabolite profiling in plants. Nat Protoc 1(1):387–396. https://doi.org/10.1038/nprot.2006.59
Lumbard K, Keefer LA, van't erve I, Carey J, Chesnick B, Butler D, Rongione M, Punt CJ, Dracopoli NC, Fijneman RJ (2022) DELFI as a real-time treatment response assessment for patients with cancer. Cancer Res 82(12_Supplement):2224–2224. https://doi.org/10.1158/1538-7445.AM2022-2224
Luo C, Wang Y, Chen Q, Han X, Liu X, Sun J, He Z (2012) Advances of paclitaxel formulations based on nanosystem delivery technology. Mini Rev Med Chem 12(5):434–444. https://doi.org/10.2174/138955712800493924
Luo J, Manning BD, Cantley LC (2003) Targeting the PI3K-Akt pathway in human cancer: rationale and promise. Cancer Cell 4(4):257–262. https://doi.org/10.1016/s1535-6108(03)00248-4
Luque-Bolivar A, Pérez-Mora E, Villegas VE, Rondón-Lagos M (2020) Resistance and overcoming resistance in breast cancer. Breast Cancer Targets Ther 12:211. https://doi.org/10.2147/BCTT.S270799
Matikas A, Kotsakis A, Apostolaki S, Politaki H, Perraki M, Kalbakis K, Nikolaou M, Economopoulou P, Hatzidaki D, Georgoulias V (2022) Detection of circulating tumour cells before and following adjuvant chemotherapy and long-term prognosis of early breast cancer. Br J Cancer 126(11):1563–1569. https://doi.org/10.1038/s41416-022-01699-5
Mazur M, Pyatchanina T (2016) The use of proteomic technologies in breast cancer research. Exp Oncol. http://dspace.nbuv.gov.ua/handle/123456789/137748
Mok TS, Wu Y-L, Kudaba I, Kowalski DM, Cho BC, Turna HZ, Castro G Jr, Srimuninnimit V, Laktionov KK, Bondarenko I (2019) Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. The Lancet 393(10183):1819–1830. https://doi.org/10.1016/S0140-6736(18)32409-7
Morganti S, Curigliano G (2020) Moving beyond endocrine therapy for luminal metastatic breast cancer in the precision medicine era: looking for new targets. Expert Rev Precis Med Drug Dev 5(1):7–22. https://doi.org/10.1080/23808993.2020.1720508
Murria R, Palanca S, de Juan I, Egoavil C, Alenda C, García-Casado Z, Juan MJ, Sánchez AB, Santaballa A, Chirivella I (2015) Methylation of tumor suppressor genes is related with copy number aberrations in breast cancer. Am J Cancer Res 5(1):375. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4300703/
Nagaraj G, Ma CX (2021) Clinical challenges in the management of hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer: a literature review. Adv Ther 38(1):109–136. https://doi.org/10.1007/s12325-020-01552-2
Nechushtan H, Vainer G, Stainberg H, Salmon AY, Hamburger T, Peretz T (2014) A phase 1/2 of a combination of cetuximab and taxane for “triple negative” breast cancer patients. The Breast 23(4):435–438. https://doi.org/10.1016/j.breast.2014.03.003
Onstenk W, Sieuwerts AM, Kraan J, Van M, Nieuweboer AJ, Mathijssen RH, Hamberg P, Meulenbeld HJ, De Laere B, Dirix LY (2015) Efficacy of cabazitaxel in castration-resistant prostate cancer is independent of the presence of AR-V7 in circulating tumor cells. Eur Urol Oncol 68(6):939–945. https://doi.org/10.1016/j.eururo.2015.07.007
Oshiro-Júnior JA, Rodero C, Hanck-Silva G, Sato MR, Alves RC, Eloy JO, Chorilli M (2020) Stimuli-responsive drug delivery nanocarriers in the treatment of breast cancer. Curr Med Chem 27(15):2494–2513. https://doi.org/10.2174/0929867325666181009120610
Patel A, Shah S (2022) Noninvasive biomarkers: emerging trends in early detection of breast cancer. Breast Cancer Bench Pers Med:125–143. https://doi.org/10.1007/978-981-19-0197-3_7
Paterlini-Brechot P, Benali NL (2007) Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett 253(2):180–204. https://doi.org/10.1016/j.canlet.2006.12.014
Pegram MD, Tan-Chiu E, Miller K, Rugo HS, Yardley DA, Liv S, Stewart SJ, Erban JK (2014) A single-arm, open-label, phase 2 study of MGAH22 (margetuximab)[fc-optimized chimeric anti-HER2 monoclonal antibody (mAb)] in patients with relapsed or refractory advanced breast cancer whose tumors express HER2 at the 2+ level by immunohistochemistry and lack evidence of HER2 gene amplification by FISH. Am J Clin Oncol. https://doi.org/10.1200/jco.2014.32.15_suppl.tps671
Pieragostino D, D’Alessandro M, di Ioia M, Di Ilio C, Sacchetta P, Del Boccio P (2015) Unraveling the molecular repertoire of tears as a source of biomarkers: beyond ocular diseases. Proteom Clin Appl 9(1–2):169–186. https://doi.org/10.1002/prca.201400084
Pinkerton JV, Thomas S (2014) Use of SERMs for treatment in postmenopausal women. J Steroid Biochem Mol Biol 142:142–154. https://doi.org/10.1016/j.jsbmb.2013.12.011
Plevritis SK, Munoz D, Kurian AW, Stout NK, Alagoz O, Near AM, Lee SJ, Van Den Broek JJ, Huang X, Schechter CB (2018) Association of screening and treatment with breast cancer mortality by molecular subtype in US women, 2000–2012. JAMA 319(2):154–164. https://doi.org/10.1001/jama.2017.19130
Ravikumar M, Rachana P (2022) Study on different approaches for breast cancer detection: a review. SN Comput Sci 3(1):1–6. https://doi.org/10.1007/s42979-021-00898-w
Rose AA, Biondini M, Curiel R, Siegel PM (2017) Targeting GPNMB with glembatumumab vedotin: current developments and future opportunities for the treatment of cancer. Pharmacol Ther 179:127–141. https://doi.org/10.1016/j.pharmthera.2017.05.010
Rouzier R, Perou CM, Symmans WF, Ibrahim N, Cristofanilli M, Anderson K, Hess KR, Stec J, Ayers M, Wagner P (2005) Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 11(16):5678–5685. https://doi.org/10.1158/1078-0432.CCR-04-2421
Salama AM, Hanna MG, Giri D, Kezlarian B, Jean M-H, Lin O, Vallejo C, Brogi E, Edelweiss M (2022) Digital validation of breast biomarkers (ER, PR, AR, and HER2) in cytology specimens using three different scanners. Mod Pathol 35(1):52–59. https://doi.org/10.1038/s41379-021-00908-5
Savard M-F, Khan O, Hunt KK, Verma S (2019) Redrawing the lines: the next generation of treatment in metastatic breast cancer. Am Soc Clin Oncol Educ Book 39:e8–e21. https://doi.org/10.1200/EDBK_237419
Scheltens P, Vijverberg E (2021) Aducanumab: appropriate use recommendations. Springer. https://doi.org/10.14283/jpad.2021.45
Schettini F, Brasó-Maristany F, Kuderer NM, Prat A (2022) A perspective on the development and lack of interchangeability of the breast cancer intrinsic subtypes. NPJ breast cancer 8(1):1–4. https://doi.org/10.1038/s41523-022-00451-9
Sharma P (2016) Biology and management of patients with triple-negative breast cancer. Oncologist 21(9):1050–1062. https://doi.org/10.1634/theoncologist.2016-0067
Shoukry M, Broccard S, Kaplan J, Gabriel E (2021) The emerging role of circulating tumor DNA in the management of breast cancer. Cancers 13(15):3813. https://doi.org/10.3390/cancers13153813
Stade A, Stade B (2017) Sélection et décryptage. J Med 377:1836–1846. https://doi.org/10.1684/ito.2017.0081
Suppan C, Brcic I, Tiran V, Mueller HD, Posch F, Auer M, Ercan E, Ulz P, Cote RJ, Datar RH (2019) Untargeted assessment of tumor fractions in plasma for monitoring and prognostication from metastatic breast cancer patients undergoing systemic treatment. Cancers 11(8):1171. https://doi.org/10.3390/cancers11081171
Tabár L, Dean PB, Tucker FL, Yen AM-F, Chen SL-S, Jen GHH, Wang JW-C, Smith RA, Duffy SW, Chen TH-H (2022) A new approach to breast cancer terminology based on the anatomic site of tumour origin: the importance of radiologic imaging biomarkers. Eur J Radiol 149:110189. https://doi.org/10.1016/j.ejrad.2022.110189
Tian J, Raffa FA, Dai M, Moamer A, Khadang B, Hachim IY, Bakdounes K, Ali S, Jean-Claude B, Lebrun J-J (2018) Dasatinib sensitises triple negative breast cancer cells to chemotherapy by targeting breast cancer stem cells. Br J Cancer 119(12):1495–1507. https://doi.org/10.1038/s41416-018-0287-3
Tong CW, Wu M, Cho WC, To KK (2018) Recent advances in the treatment of breast cancer. Front Oncol 8:227. https://doi.org/10.3389/fonc.2018.00227
Veeraraghavan J, De Angelis C, Mao R, Wang T, Herrera S, Pavlick A, Contreras A, Nuciforo P, Mayer I, Forero A (2019) A combinatorial biomarker predicts pathologic complete response to neoadjuvant lapatinib and trastuzumab without chemotherapy in patients with HER2+ breast cancer. Ann Oncol 30(6):927–933. https://doi.org/10.1093/annonc/mdz076
Waks AG, Winer EP (2019) Breast cancer treatment: a review. JAMA 321(3):288–300. https://doi.org/10.1001/jama.2018.19323
Watanabe K, Nakamura Y, Low S-K (2021) Clinical implementation and current advancement of blood liquid biopsy in cancer. J Hum Genet 66(9):909–926. https://doi.org/10.1038/s10038-021-00939-5
Xuhong JC, Qi XW, Zhang Y, Jiang J (2019) Mechanism, safety and efficacy of three tyrosine kinase inhibitors lapatinib, neratinib and pyrotinib in HER2-positive breast cancer. Am J Cancer Res 9(10):2103. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6834479/
Yadav BS, Chanana P, Jhamb S (2015) Biomarkers in triple negative breast cancer: a review. World J Clin Oncol 6(6):252. https://doi.org/10.5306/wjco.v6.i6.252
Yang SX, Polley EC (2019) Systemic treatment and radiotherapy, breast cancer subtypes, and survival after long-term clinical follow-up. Breast Cancer Res Treat 175(2): 287–295. https://doi.org/10.1007/s10549-019-05142-x
Yoon M-S (2017) The role of mammalian target of rapamycin (mTOR) in insulin signaling. Nutrients 9(11):1176. https://doi.org/10.3390/nu9111176
Yu KD, Liu GY, Chen CM, Li JW, Wu J, Lu JS, Shen ZZ, Shao ZM (2013) Weekly paclitaxel/carboplatin/trastuzumab therapy improves pathologic complete remission in aggressive HER2-positive breast cancers, especially in luminal-B subtype, compared with a once-every-3-weeks schedule. Oncologist 18(5):511–517. https://doi.org/10.1634/theoncologist.2012-0057
Zhang F, Ni Z-J, Ye L, Zhang Y-Y, Thakur K, Cespedes-Acuña CL, Han J, Zhang J-G, Wei Z-J (2021) Asparanin A inhibits cell migration and invasion in human endometrial cancer via Ras/ERK/MAPK pathway. Food Chem Toxicol 150:112036. https://doi.org/10.1016/j.fct.2021.112036
Zhang S, Yu D (2012) Targeting Src family kinases in anti-cancer therapies: turning promise into triumph. Trends Pharmacol Sci 33(3):122–128. https://doi.org/10.1016/j.jbi.2021.103764
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2024 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
De, A., Patel, S., Gowthamarajan, K. (2024). Next-Generation Therapies for Breast Cancer. In: Madhusudhan, A., Purohit, S.D., Prasad, R., Husen, A. (eds) Functional Smart Nanomaterials and Their Theranostics Approaches. Smart Nanomaterials Technology. Springer, Singapore. https://doi.org/10.1007/978-981-99-6597-7_5
Download citation
DOI: https://doi.org/10.1007/978-981-99-6597-7_5
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-99-6596-0
Online ISBN: 978-981-99-6597-7
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)