Abstract
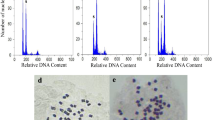
Garden cress (Lepidium sativum L., Brassicaceae) is one of the most popular leafy vegetables which is widely used, and has also various medicinal properties and industrial usage. Small and very delicate leaves of this short period and fast growing species cause lots of crop losses along production to consumption; so it was supposed that increase in thickness and size of the leaves via induction of polyploidy possibly will improve post-harvest quality. Primary trial proved that seed treatments, via immersion of dry and wet seeds in different concentrations and durations of colchicine, were completely ineffective. Thereafter dropping method was conducted on apical bud of cotyledon and two true leaf stages with different concentrations of colchicine (0, 0.05, 0.1, 0.2, 0.5 and 0.75% w/v). Treatment on cotyledon stage was not fruitful because of sensitivity to colchicine and dying of small seedlings; but apical bud treatment in two true leaf stage resulted in inducing some polyploid plants. The best result was obtained by 0.5% colchicine concentration, inducing 9.33% tetraploid plants. Chromosome counting and flowcytometric analysis of morphologically putative plants confirmed chromosome doubling in garden cress from 2n = 2x = 16 to 2n = 4x = 32. Tetraploid plants comparing diploid ones specified by increasing in leaf size and thickness, stem diameter, stomata size, number of chloroplasts in stomata guard cells, seed weight and on the contrary, decreasing in stomata count and height of plants, percentage of seed germination and also germination rate.
Key message
In this research, we have tested various methods and different levels of colchicine for the polyploidy induction in garden cress, and the results of polyploidy induction have been studied.










Similar content being viewed by others
References
Adaniya S, Shira D (2001) In vitro induction of tetraploid ginger (Zingiber officinali Roscoe) and its pollen fertility and germinability. Sci Hortic-Amsterdam 88:277–287
Banyai W, Sangthong R, Karaket N, Inthima P, Mii M, Supaibulwatana K (2010) Over production of artemisinin in tetraploid Artemisia annua L. Plant Biotechnol 27:427–433
Beyaz R, Alizadeh B, Gürel S, Oscan SF, Yıldız M (2013) Sugar beet (Beta vulgaris L.) growth at different ploidy levels. Caryologia 66:90–95
Blakeslee AF, Avery AG (1937) Methods of inducing doubling of chromosomes in plants: by treatment with colchicine. J Hered 28:393–411
Bouvier L, Pillon FR, Lespinasse Y (1994) Oryzalin as an efficient agent for chromosome doubling of haploid apple shoots in vitro. Plant Breed 113:343–346
Bretagnolle F, Thompson JD, Lumaret R (1995) The influence of seed size variation on seed germination and seedling vigour in diploid and tetraploid Dactylis glomerata L. Ann Bot-London 76:607–615
Breuer C, Stacey NJ, West CE, Zhao Y, Chory J, Tsukaya H, Azumi Y, Maxwell A, Roberts K, Sugimoto-Shirasu K (2007) BIN4, a novel component of the plant DNA topoisomerase VI complex, is required for endo-reduplication in Arabidopsis. Plant Cell 19:3655–3668
Chakraborti SP, Vijayan K, Roy BN, Qadri SMH (1998) In vitro induction of tetraploidy in mulberry (Morus alba L.). Plant Cell Rep 17:799–803
Dhamayanthi KPM, Gotmare V (2010) Induction of polyploidy in two diploid wild cotton (G. armourianum and G. aridum) species by colchicine treatment. Electron J Plant Breed 1(4):966–972
Dhawan OP, Lavania UC (1996) Enhancing the productivity of secondary metabolites via induced polyploidy: a review. Euphytica 87:81–89
Dhooghe E, Van Laere K, Eeckhaut T, Leus L, Van Huylenbroeck J (2011) Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult 104:359–373
Dolezel J, Bartos J (2005) Plant DNA flow cytometry and estimation of nuclear genome size. Ann Bot-London 95:99–110
Dolezel J, Sgorbati S, Lucretti S (1992) Comparison of three DNA fluorochromes for flow cytometric estimation of nuclear DNA content in plants. Physiol Plant 85:625–631
Dolezel J, Bartos J, Voglmayr H, Greilhuber J (2003) Nuclear DNA content and genome size of trout and human. Cytometry 51:127–129
Eddouks M, Maghrani M, Zeggwagh NA, Michel JB (2005) Study of the hypoglycaemic activity of Lepidium sativum L. aqueous extract in normal and diabetic rats. J Ethnopharmacol 97:391–395
Eliasova A, Munzbergova Z (2014) Higher seed size and germination rate may favour autotetraploids of Vicia cracca L. (Fabaceae). Biol J Linn Soc 113:57–73
Gao SL, Zhu DN, Cai ZH, Xu DR (1996) Autotetraploid plants from colchicine treated bud culture of Salvia miltiorrhiza. Plant Cell Tissue Organ Cult 47:73–77
Gilani AH, Rehman NU, Mehmood MH, AlKharfy KM (2012) Species differences in the antidiarrheal and antispasmodic activities of Lepidium sativum and insight into underlying mechanisms. Phytother Res 27(7):1086–1094
Grubben GJH, Denton OA (2004) Plant resources of Tropical Africa, 2. Vegetables. PROTA Foundation, Wageningen
Hernandez Bermejo J, EstebanLeon J (1994) Neglected crops: 1492 from a different perspective. Food and Agriculture Organization of the United Nations, Rome
Huaman Z (1995) Tecnicas citologicas para determinar el numero cromosomico y la fertilidad de las papas. Centro Internacional de la Papa, Lima, p 18
Jaskani MJ, Kwon SW, Kim DH (2005) Comparative study on vegetative, reproductive and qualitative traits of seven diploid and tetraploid watermelon lines. Euphytica 145:259–268
Jaskani MJ, Kwon SW, Kim DH, Abbas H (2006) Seed treatments and orientation affects germination and seedling emergence in tetraploid watermelon. Pak J Bot 38:89
Jesus-Gonzalea LD, Weathers PJ (2003) Tetraploid Artemisia annua hairy roots produce more artemisinin than diploids. Plant Cell Rep 21:809–813
Johnston JS, Pepper AE, Hall AE, Chen ZJ, Hodnett G, Drabek J, Lopez R, Price HJ (2005) Evolution of genome size in Brassicaceae. Ann Bot-London 95:229–235
Khosravi P, Kermani MJ, Nematzadeh GA, Bihamta MR, Yokoya K (2008) Role of mitotic inhibitors and genotype on. chromosome doubling of Rosa. Euphytica 160:267–275
Lehrer JM, Mark HB, Lubell JD (2008) Induction of tetraploidy in meristematically active seeds of Japanese barberry (Berberis thunbergii var. Atropurpurea) through exposure to colchicine and oryzalin. Sci Hortic-Amsterdam 119:67–71
Levin DA (2002) The role of chromosomal change in plant evolution. Oxford University Press, Oxford
Liu G, Li Z, Bao M (2007) Colchicine-induced chromosome doubling in Platanus acerifolia effect on plant morphology. Euphytica 157:145–154
Loureiro J, Rodriguez E, Dolezel J, Santos C (2007) Two new nuclear isolation buffers for plant DNA flow cytometry: a test with 37 species. Ann Bot-London 100(4):875–888
Madon M, Clyde MM, Hashim H, Mohdyusuf Y, Mat H, Saratha S (2005) Polyploidy induction of oil palm through colchicine and oryzalin treatments. J Oil Palm Res 17:110–123
Majdi M, Karimzadeh G, Malboobi MA, Omidbaigi R, Mirzaghaderi G (2010) Induction of tetraploidy to feverfew (Tanacetum parthenium Schulz-Bip.): morphological, physiological, cytological and phytochemical changes. Hortscience 45(1):1–6
Mathura S, Fossey A, Beck S (2006) Comparative study of chlorophyll content in diploid and tetraploid black Wattle (Acacia mearnsii). Forestry 79(4):381–388
Moeini A, Abdoli M, NaghdiBadi HA (2013) Morphological, physiological, cytological and phytochemical studies in diploid and colchicine-induced tetraploid plants of Echinacea purpurea. Acta Physiol Plant 35:2075–2083
Molin WT, Meyers SP, Baer GR, Schrader LE (1982) Ploidy effects of isogenic populations of alfalfa II. Photosynthesis, chloroplast number, ribulose-1,5-bisphosphate carboxylase, chlorophyll, and DNA in protoplasts. Plant Physiol 70:1710–1714
Nakano M, Nomizu T, Mizunashi K, Suzuki M, Mori S, Kuwayama S, Hayashi M, Umehara H, Oka E, Kobayashi H (2006) Somaclonal variation in Tricyrtis hirta plants regenerated from 1-year-old embryogenic callus cultures. Sci Hortic 110:366–371
Nehdi IA, Sbihi H, Tan CP, Al-Resayes SI (2012) Garden cress (Lepidium sativum L.) seed oil as a potential feedstock for biodiesel production. Bioresour Technol 126:193–197
Noori SA, Norouzi M, Karimzadeh G, Shirkool K, Niazian M (2017) Effect of colchicine-induced polyploidy on morphological characteristics and essential oil composition of ajowan (Trachyspermum ammi L.). Plant Cell Tissue Organ Cult 130:543–551
Oates KM, Ranney TG, Touchell DH (2012) Influence of induced polyploidy on fertility and morphology of Rudbeckia species and hybrids. Hortscience 47:1217–1221
Omidbaigi R, Mirzaeea M, Hassani ME, Sedghi-Moghadam M (2010) Induction and identification of polyploidy in basil (Ocimum basilicum) medicinal plant by colchicine treatment. Int J Plant Prod 4(2):87–98
Pansuksan K, Sangthong R, Nakamura I, Mii M, Supaibulwatana K (2014) Tetraploid induction of Mitracar pushirtus by colchicine and its characterization including antibacterial activity. Plant Cell Tissue Organ Cult 117:381–391
Petersen KK, Hagberg P, Kristiansen K (2003) Colchicine and oryzalin mediated chromosome doubling in different genotypes of Miscanthus sinensis. Plant Cell Tissue Organ Cult 73:137–146
Pourmohammadi P, Moieni A, Ebrahimi A, Javidfar F (2012) Doubled haploid plants following colchicine treatment of microspore-derived embryos of oilseed rape (Brassica napus). Plant Cell Tissue Organ Cult 108(2):251–256
Quan K, Guolu L, Qigao G, Xiaolin L (2004) Polyploid induction of Arctium lappa by colchicine. Plant Physiol Commun 40:157–158
Shao J, Chen C, Deng X (2003) In vitro induction of tetraploid in pomegranate (Punica granatum). Plant Cell Tissue Organ Cult 75:241–246
Sharma AK, Sikka K (1976) Chromosome studies in Cruciferae. Res Bull Univ Calcutta Cytogenetics Lab 3:33–34
Sikdar AK, Jolly MS (1994) Induced polyploidy in mulberry (Morus spp.): induction of tetraploids. Sericologia 34:105–116
Smith S, Weyers JDB, Berry WG (1989) Variation in stomatal characteristics over the lower surface of Commelina communis leaves. Plant Cell Environ 12:653–659
Stanys V, Weckman A, Staniene G, Duchovskis P (2006) In vitro induction of polyploidy in Japanese quince (Chaenomeles japonica). Plant Cell Tissue Organ Cult 84:263–268
Stebbins GL (1971) Chromosomal evolution in higher plants. Edward Arnold, London
Sugiyama S (2005) Polyploidy and cellular mechanisms changing leaf size: comparison of diploid and autotetraploid populations in two species of Lolium. Ann Bot-London 96:931–938
Tang ZQ, Chen DL, Song ZJ, He YC, Cai DT (2010) In vitro induction and identification of tetraploid plants of Paulownia tomentosa. Plant Cell Tissue Organ Cult 102:213–220
Tavan M, Mirjalili MH, Karimzadeh G (2015) In vitro polyploidy induction: changes in morphological, anatomical and phytochemical characteristics ofThymuspersicus (Lamiaceae). Plant Cell Tissue Organ Cult 122:573–583
Thao NTP, Ureshino K, Miyajima I, Ozaki Y, Okubo H (2003) Induction of tetraploids in ornamental Alocasia through colchicine and oryzalin treatments. Plant cell Tissue Organ Cult 72:19–25
Wadhwa S, Panwar MS, Agrawal A, Saini N, Patidar N (2012) A review on pharmacognostical study of Lepidium sativum. Adv Res Pharm Biol 2(4):316–323
Warner DA, Edwards GE (1989) Effects of polyploidy on photosynthetic rates, photosynthetic enzymes, contents of DNA, chlorophyll, and sizes and numbers of photosynthetic cells in the C4 dicot Atriplex confertifolia. Plant Physiol 91:1143–1151
Ye YM, Tong J, Shi XP, Yuan W, Li GR (2010) Morphological and cytological studies of diploid and colchicine-induced tetraploid lines of crape myrtle (Lagerstroemia indica L.). Sci Hortic-Amsterdam 124:95–101
Zhang XY, Hu CG, Yao JL (2010) Tetraploidization of diploid Dioscorea results in activation of the antioxidant defense system and increased heat tolerance. J Plant Physiol 167:88–94
Author information
Authors and Affiliations
Contributions
AA and ML conducted the experiments and wrote the manuscript, MN in the cytogenetic section helped and GK helped to improve the manuscript and Flow cytometric study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest to disclose.
Ethical approval
The experiments were performed according to the current laws of Islamic Republic of Iran.
Additional information
Communicated by Alison M.R. Ferrie.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aqafarini, A., Lotfi, M., Norouzi, M. et al. Induction of tetraploidy in garden cress: morphological and cytological changes. Plant Cell Tiss Organ Cult 137, 627–635 (2019). https://doi.org/10.1007/s11240-019-01596-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-019-01596-5