Abstract
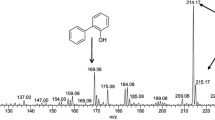
Metabolically microorganisms are diverse, and they are capable of transforming almost every known group of chemical compounds present in coal and oil in various forms. In this milieu, one of the important microbial metabolic processes is the biodesulfurization [cleavage of carbon–sulfur (C–S) bond] of thiophenic compounds, such as dibenzothiophene (DBT), which is the most abundant form of organic sulfur present in fossil fuels. In the current study, ten newly isolated bacterial isolates, designated as species of genera Gordonia, Amycolatopsis, Microbacterium and Mycobacterium, were enriched from different samples in the presence of DBT as a sole source of organic sulfur. The HPLC analysis of the DBT grown cultures indicated the consumption of DBT and accumulation of 2-hydroxybiphenyl (2-HBP). Detection of 2-HBP, a marker metabolite of 4S (sulfoxide–sulfone–sulfinate–sulfate) pathway, suggested that the newly isolated strains harbored metabolic activity for DBT desulfurization through the cleavage of C–S bond. The maximum 2-HBP formation rate was 3.5 µmol/g dry cell weight (DCW)/h. The phylogenetic analysis of the new isolates showed that they had diverse distribution within the phylogenetic tree and formed distinct clusters, suggesting that they might represent strains of already reported species or they were altogether new species. Estimates of evolutionary divergence showed high level of nucleotide divergence between the isolates within the same genus. The new isolates were able to use a range of heterocyclic sulfur compounds, thus making them suitable candidates for a robust biodesulfurization system for fossil fuels.




Similar content being viewed by others
References
Abin Fuentes A, Leung JC, Mohamed MES, Wang DIC, Prather KLJ (2014) Rate limiting step analysis of the microbial desulfurization of dibenzothiophene in a model oil system. Biotechnol Bioeng 111:876–884
Aggarwal S, Karimi IA, Ivan GR (2013) In silico modeling and evaluation of Gordonia alkanivorans for biodesulfurization. Mol BioSyst 9:2530–2540
Akhtar N, Ghauri MA, Anwar MA, Akhtar K (2009) Analysis of the dibenzothiophene metabolic pathway in a newly isolated Rhodococcus spp. FEMS Microbiol Lett 301:95–102
Alves L, Salgueiro R, Rodrigues C, Mesquita E, Matos J, Girio FM (2005) Desulfurization of dibenzothiophene, benzothiophene, and other thiophene analogs by a newly isolated bacterium, Gordonia alkanivorans strain 1B. Appl Biochem Biotechnol 120:199–208
Arenskötter M, Bröker D, Steinbüchel A (2004) Biology of the metabolically diverse genus Gordonia. Appl Environ Microbiol 70:3195–3204
Ausbel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1995) Short protocols in molecular biology, 3rd edn. Wiley, New York
Bhatia S, Sharma DK (2010) Biodesulfurization of dibenzothiophene, its alkylated derivatives and crude oil by a newly isolated strain Pantoea agglomerans D23W3. Biochem Eng J 50:104–109
Boniek D, Figueiredo D, Pylro VS, Duarte GF (2010) Characterization of bacterial strains capable of desulphurization in soil and sediment samples from Antarctica. Extremophiles 14:475–481
Buzanello EB, Rezende RP, Sousa FMO, Marques ELS, Loguercio LL (2014) A novel Bacillus pumilus related strain from tropical landfarm soil is capable of rapid dibenzothiophene degradation and biodesulfurization. BMC Microbiol 14(1):257
Chen H, Zhang WJ, Chen JM, Cai YB, Li W (2008) Desulfurization of various organic sulfur compounds and the mixture of DBT+ 4, 6-DMDBT by Mycobacterium sp. ZD-19. Bioresour Technol 99:3630–3634
Chen H, Cai YB, Zhang WJ, Li W (2009) Methoxylation pathway in biodesulfurization of model organosulfur compounds with Mycobacterium sp. Bioresour. Technol 100:2085–2087
Eriksen J (2008) Soil sulfur cycling in temperate agricultural system. In: Sulfur: a missing link between soils, crops, and nutrition. ASA-CSSA-SSSA
Gallagher JR, Olson ES, Stanley DC (1993) Microbial desulfurization of dibenzothiophene: a sulfur-specific pathway. FEMS Microbiol Lett 107:31–35
Gupta N, Roychoudhury PK, Deb JK (2005) Biotechnology of desulfurization of diesel: prospects and challenges. Appl Microbiol Biotechnol 66:356–366
Gurtler V, Mayall BC, Seviour R (2004) Can whole genome analysis refine the taxonomy of the genus Rhodococcus? FEMS Microbiol Rev 28:377–403
Izumi Y, Ohshiro T, Ogino H, Hine Y (1994) Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Appl Environ Microbiol 60(1):223–226
Kayser KJ, Cleveland L, Park HS, Kwak JH, Kolhatkar A, Kilbane JJ II (2002) Isolation and characterization of a moderate thermophile, Mycobacterium phlei GTIS10, capable of dibenzothiophene desulfurization. Appl Microbiol Biotechnol 59:737–746
Kertesz MA (2000) Riding the sulfur cycle–metabolism of sulfonates and sulfate esters in Gram-negative bacteria. FEMS Microbiol Rev 24:135–175
Kilbane JJ (1990) Sulfur-specific microbial-metabolism of organic-compounds. Resour Conserv Recycl 3(2–3):69–79
Kilbane JJ (1991) Bacterial produced extracts and enzymes for cleavage of organic C–S bonds. European Patent No. 0445896A2
Kilbane JJ (2006) Microbial biocatalyst developments to upgrade fossil fuels. Curr Opin Biotech 17:305–314
Li W, Zhang Y, Wang MD, Shi Y (2005) Biodesulfurization of dibenzothiophene and other organic sulfur compounds by a newly isolated Microbacterium strain ZD-M2. FEMS Microbiol Lett 247:45–50
Li F, Zhang Z, Feng J, Cai X, Xu P (2007) Biodesulfurization of DBT in tetradecane and crude oil by a facultative thermophilic bacterium Mycobacterium goodii X7B. J Biotechnol 127:222–228
Maass D, Todescato D, Moritz DE, Oliveira JV, Oliveira D, Ulson de Souza AA, Guelli Souza SM (2015) Desulfurization and denitrogenation of heavy gas oil by Rhodococcus erythropolis ATCC 4277. Bioprocess Biosyst Eng 38(8):1447–1453
Mohamed ME-S, Al-Yacoub ZH, Vedakumar JV (2015) Biocatalytic desulfurization of thiophenic compounds and crude oil by newly isolated bacteria. Front Microbiol 6:1–12
Monticello DJ (2000) Biodesulfurization and the upgrading of petroleum distillates. Curr Opin Biotechnol 11:540–546
Monticello DJ, Finnerty WR (1985) Microbial desulfurization of fossil fuels. Annu Rev Microbiol 39:371–389
Nekodzuka S, Nakajima-Kambe T, Nomura N, Lu J, Nakahara T (1997) Specific desulfurization of dibenzothiophene by Mycobacterium sp. strain G3. Biocatal Biotransform 15:17–27
Nuhu AA (2013) Bio-catalytic desulfurization of fossil fuels: a mini review. Rev Environ Sci Biotechnol 12:9–23
Ohshiro T, Suzuki K, Izumi Y (1996) Regulation of dibenzothiophene degrading enzyme activity of Rhodococcus erythropolis D-1. J Ferment Bioeng 81:121–124
Oldfield C, Pogrebinsky O, Simmonds J, Olson ES, Kulpa CF (1997) Elucidation of the metabolic pathway for dibenzothiophene desulphurization by Rhodococcus sp. strain IGTS8 (ATCC 53968). Microbiology 143:2961–2973
Rashtchi M, Mohebali GH, Akbarnejad MM, Towfighi J, Rasekh B, Keytash A (2006) Analysis of biodesulfurization of model oil system by the bacterium, strain RIPI-22. Biochem Eng J 29:169–173
Soleimani M, Bassi A, Margaritis A (2007) Biodesulphurization of refractory organic sulfur compounds in fossil fuels. Biotechnol Adv 25(6):570–596
Stackebrandt E, Goebel BM (1994) A place for DNA-DNA reassociation and 16S ribosomal-RNA sequence-analysis in the present species definition in bacteriology. Int J Syst Bacteriol 44:846–849
Stoner DL, Wey JE, Barrett KB, Jolley JG, Wright RB, Dugan PR (1990) Modification of water-soluble coal-derived products by dibenzothiophene-degrading microorganisms. Appl Environ Microbiol 56:2667–2676
Tamura K, Nei M (1993) Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol 10:512–526
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Tanaka Y, Matsui T, Konishi J, Maruhashi K, Kurane R (2002) Biodesulfurization of benzothiophene and dibenzothiophene by a newly isolated Rhodococcus strain. Appl Microbiol Biotechnol 59:325–328
Ting MA, Shanshan LI, Guoqiang LI, Renjing WANG, Fenglai LIANG, Rulin LIU (2006) Cloning and expressing DBT (dibenzothiophene) monooxygenase gene (dsz C) from Rhodococcus sp. DS-3 in Escherichia coli. Front Biol China 4:375–380
Wang W, Ma T, Lian K, Zhang Y, Tian H, Ji K, Li G (2013) Genetic analysis of benzothiophene biodesulfurization pathway of Gordonia terrae strain C-6. PLoS One 8(12):e84386
Acknowledgments
This research work was supported by the International Foundation for Science, Stockholm, Sweden, through a grant to Dr. Nasrin Akhtar (Research Grant Agreement No. F/5379-1).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interests.
Additional information
Communicated by Matthias Boll.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Akhtar, N., Ghauri, M.A. & Akhtar, K. Dibenzothiophene desulfurization capability and evolutionary divergence of newly isolated bacteria. Arch Microbiol 198, 509–519 (2016). https://doi.org/10.1007/s00203-016-1209-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-016-1209-5